Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss.1 Studies have found increased risks of gastrointestinal adverse events (biliary disease,2 pancreatitis,3 bowel obstruction,4 and gastroparesis5) in patients with diabetes.2-5 Because such patients have higher baseline risk for gastrointestinal adverse events, risk in patients taking these drugs for other indications may differ. Randomized trials examining efficacy of GLP-1 agonists for weight loss were not designed to capture these events2 due to small sample sizes and short follow-up. We examined gastrointestinal adverse events associated with GLP-1 agonists used for weight loss in a clinical setting.
We used a random sample of 16 million patients (2006-2020) from the PharMetrics Plus for Academics database (IQVIA), a large health claims database that captures 93% of all outpatient prescriptions and physician diagnoses in the US through the International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10. In our cohort study, we included new users of semaglutide or liraglutide, 2 main GLP-1 agonists, and the active comparator bupropion-naltrexone, a weight loss agent unrelated to GLP-1 agonists. Because semaglutide was marketed for weight loss after the study period (2021), we ensured all GLP-1 agonist and bupropion-naltrexone users had an obesity code in the 90 days prior or up to 30 days after cohort entry, excluding those with a diabetes or antidiabetic drug code.
Bạn đang xem: Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
Patients were observed from first prescription of a study drug to first mutually exclusive incidence (defined as first ICD-9 or ICD-10 code) of biliary disease (including cholecystitis, cholelithiasis, and choledocholithiasis), pancreatitis (including gallstone pancreatitis), bowel obstruction, or gastroparesis (defined as use of a code or a promotility agent). They were followed up to the end of the study period (June 2020) or censored during a switch. Hazard ratios (HRs) from a Cox model were adjusted for age, sex, alcohol use, smoking, hyperlipidemia, abdominal surgery in the previous 30 days, and geographic location, which were identified as common cause variables or risk factors.6 Two sensitivity analyses were undertaken, one excluding hyperlipidemia (because more semaglutide users had hyperlipidemia) and another including patients without diabetes regardless of having an obesity code. Due to absence of data on body mass index (BMI), the E-value was used to examine how strong unmeasured confounding would need to be to negate observed results, with E-value HRs of at least 2 indicating BMI is unlikely to change study results. Statistical significance was defined as 2-sided 95% CI that did not cross 1. Analyses were performed using SAS version 9.4. Ethics approval was obtained by the University of British Columbia’s clinical research ethics board with a waiver of informed consent.
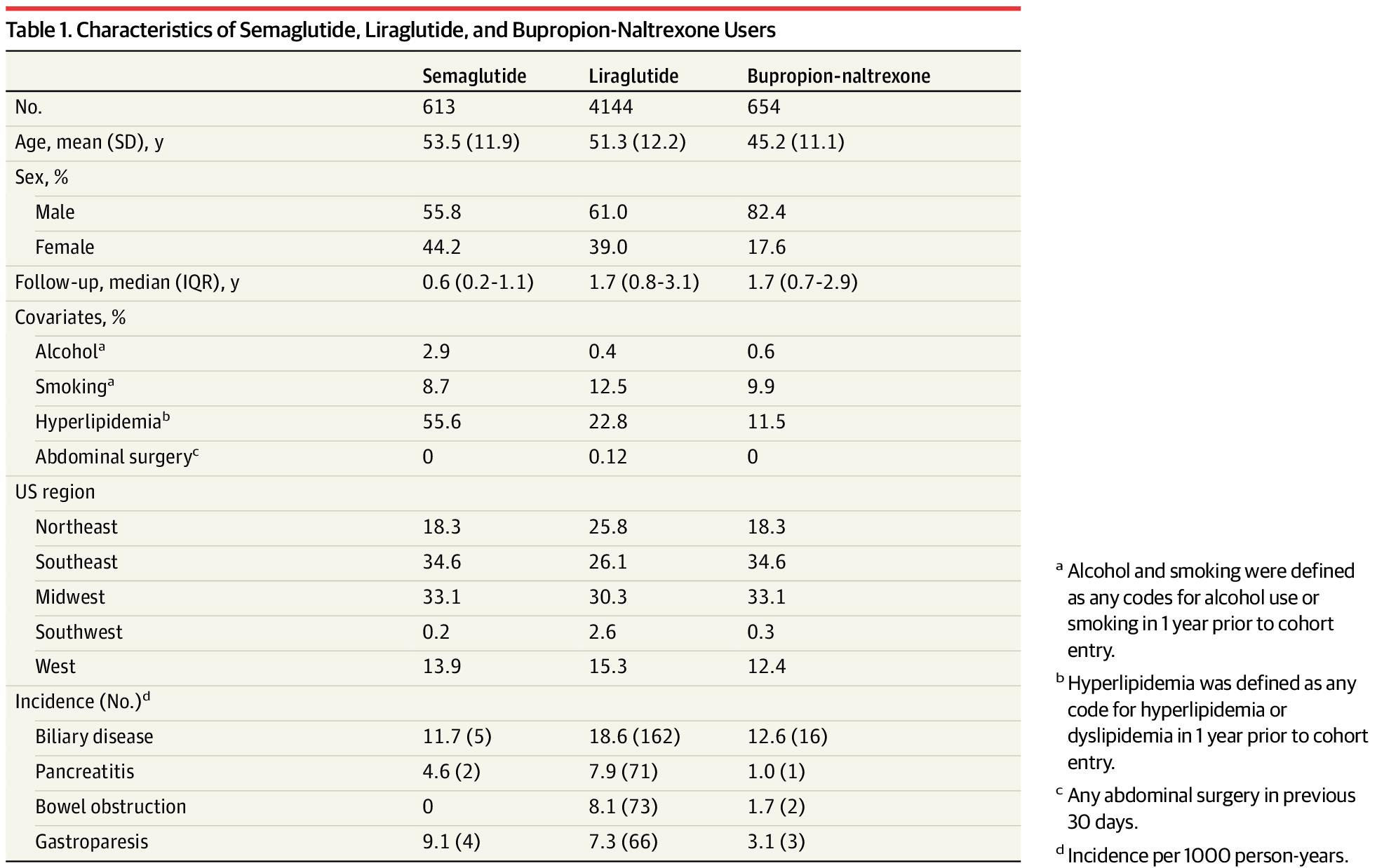
Our cohort included 4144 liraglutide, 613 semaglutide, and 654 bupropion-naltrexone users. Incidence rates for the 4 outcomes were elevated among GLP-1 agonists compared with bupropion-naltrexone users (Table 1). For example, incidence of biliary disease (per 1000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for bupropion-naltrexone and 4.6, 7.9, and 1.0, respectively, for pancreatitis.
Use of GLP-1 agonists compared with bupropion-naltrexone was associated with increased risk of pancreatitis (adjusted HR, 9.09 [95% CI, 1.25-66.00]), bowel obstruction (HR, 4.22 [95% CI, 1.02-17.40]), and gastroparesis (HR, 3.67 [95% CI, 1.15-11.90) but not biliary disease (HR, 1.50 [95% CI, 0.89-2.53]). Exclusion of hyperlipidemia from the analysis did not change the results (Table 2). Inclusion of GLP-1 agonists regardless of history of obesity reduced HRs and narrowed CIs but did not change the significance of the results (Table 2). E-value HRs did not suggest potential confounding by BMI.
This study found that use of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
Given the wide use of these drugs, these adverse events, although rare, must be considered by patients who are contemplating using the drugs for weight loss because the risk-benefit calculus for this group might differ from that of those who use them for diabetes. Limitations include that although all GLP-1 agonist users had a record for obesity without diabetes, whether GLP-1 agonists were all used for weight loss is uncertain.
Accepted for Publication: September 11, 2023.
Xem thêm : Hiatal Hernia Repair – After Gastric Sleeve
Published Online: October 5, 2023. doi:10.1001/jama.2023.19574
Correction: This article was corrected on December 21, 2023, to update the full name of the database used.
Corresponding Author: Mahyar Etminan, PharmD, MSc, Faculty of Medicine, Departments of Ophthalmology and Visual Sciences and Medicine, The Eye Care Center, University of British Columbia, 2550 Willow St, Room 323, Vancouver, BC V5Z 3N9, Canada (etminanm@mail.ubc.ca).
Author Contributions: Dr Etminan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sodhi, Rezaeianzadeh, Etminan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sodhi, Rezaeianzadeh, Etminan.
Critical review of the manuscript for important intellectual content: All authors.
Xem thêm : Behavioral Health at Ferguson Medical Group
Statistical analysis: Kezouh.
Obtained funding: Etminan.
Administrative, technical, or material support: Sodhi.
Supervision: Etminan.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was funded by internal research funds from the Department of Ophthalmology and Visual Sciences, University of British Columbia.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement.
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on November 23, 2024 8:55 am