Background In patients undergoing dialysis, therapy with calcitriol or paricalcitol or other vitamin D agents is associated with reduced mortality. Observational data suggests that low 25-hydroxyvitamin D levels (25[OH]D) are associated with diabetes mellitus, hypertension, and cancers. However, whether low serum 25(OH)D levels are associated with mortality in the general population is unknown.
Methods We tested the association of low 25(OH)D levels with all-cause, cancer, and cardiovascular disease (CVD) mortality in 13 331 nationally representative adults 20 years or older from the Third National Health and Nutrition Examination Survey (NHANES III) linked mortality files. Participant vitamin D levels were collected from 1988 through 1994, and individuals were passively followed for mortality through 2000.
Bạn đang xem: 25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population
Results In cross-sectional multivariate analyses, increasing age, female sex, nonwhite race/ethnicity, diabetes, current smoking, and higher body mass index were all independently associated with higher odds of 25(OH)D deficiency (lowest quartile of 25(OH)D level, <17.8 ng/mL [to convert to nanomoles per liter, multiply by 2.496]), while greater physical activity, vitamin D supplementation, and nonwinter season were inversely associated. During a median 8.7 years of follow-up, there were 1806 deaths, including 777 from CVD. In multivariate models (adjusted for baseline demographics, season, and traditional and novel CVD risk factors), compared with the highest quartile, being in the lowest quartile (25[OH]D levels <17.8 ng/mL) was associated with a 26% increased rate of all-cause mortality (mortality rate ratio, 1.26; 95% CI, 1.08-1.46) and a population attributable risk of 3.1%. The adjusted models of CVD and cancer mortality revealed a higher risk, which was not statistically significant.
Conclusion The lowest quartile of 25(OH)D level (<17.8 ng/mL) is independently associated with all-cause mortality in the general population.
Several studies have suggested that 25-hydroxyvitamin (25[OH]D) deficiency is an unrecognized contributor to the development of cardiovascular disease (CVD), cancer, and mortality. 1,25-Dihydroxyvitamin D affects the renin-angiotensin system,1 is associated with cardiac myocyte hypertrophy,2 and has anti-inflammatory effects,3 all of which may influence CVD risk.4 In addition, 1,25-dihydroxyvitamin D has antiproliferative activity, which may influence cancer risk.5 Therapy with calcitriol or paricalcitol or other vitamin D agents (activated vitamin D therapy) is associated with lower mortality in those with end-stage renal disease.6-10 In addition, low 25(OH)D levels have been associated with multiple CVD risk factors in the Third National Health and Nutrition Examination Survey (NHANES III) population11 as well as with hypertension,12 congestive heart failure,13 cancer,14 and diabetes mellitus.15,16 Low 25(OH)D levels in incident hemodialysis patients have recently been shown to be associated with all-cause mortality.17 A meta-analysis of 18 randomized clinical trials of vitamin D supplementation in mostly older individuals found that randomization to vitamin D supplementation was associated with lower all-cause mortality.18 Despite these suggested associations, we found no published studies evaluating the relationship between 25(OH)D levels and mortality risk in the general population.
The optimal level of 25(OH)D has been suggested to be 30 ng/mL or greater (to convert to nanomoles per liter, multiply by 2.496),19,20 a level associated with maximal suppression of parathyroid hormone and reduced fracture rates and postulated to be associated with better health outcomes. Approximately 41% of men and 53% of women in the United States, however, have levels of 25(OH)D below 28 ng/mL.21
We hypothesized that low serum 25(OH)D level is a risk factor for cancer, CVD, and all-cause mortality among adults in the United States. The NHANES III linked mortality data set provides an excellent opportunity to test the association of 25(OH)D deficiency with mortality in a large, multiracial sample of the general population.
The Third National Health and Nutrition Examination Survey is a nationwide probability sample of noninstitutionalized civilian persons conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. Details regarding informed consent and statistical methods are outlined elsewhere.22 We restricted our analyses to adults 20 years or older who had a physical examination and laboratory testing at baseline (October 1988-October 1994) and for whom vital status information was known during the follow-up period (through December 31, 2000). Non-Hispanic blacks, Mexican Americans, and elderly persons were oversampled in NHANES III to allow for more precise estimates in these groups.23 To ensure comparable conditions at each NHANES survey site, northern states were surveyed during the summer and southern states were surveyed during the winter. Participants were excluded if they did not have complete data on the study variables of interest. The remaining 13 331 participants represent approximately 175 million people in the United States. The institutional review boards at the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, and the Albert Einstein College of Medicine, Bronx, New York, determined this analysis to be exempt.
Interview questions, physical examination, and laboratory values, such as C-reactive protein, glucose, albumin, creatinine, and lipids levels, were assessed in all study participants at baseline during 1988 through 1994 and processed per standard protocol.22 Laboratory measurements occurred during either morning, afternoon, or evening sessions. Participants were asked to fast 12 hours before the morning examination or 6 hours for the afternoon or evening examination. To minimize hemolysis of blood samples, which will affect serum calcium but not serum 25(OH)D levels, all phlebotomists were certified and completed training in standardized laboratory procedures annually. Serum 25(OH)D was measured using the Diasorin radioimmunoassay (RIA) kit (Diasorin, Stillwater, Minnesota) on frozen serum (< −20°C) between February 1994 and December 1995 (total coefficients of variation from quality control samples, 13%-19%). The RIA kit was calibrated using high-performance liquid chromatography-purified 25(OH)D every 6 months. Race/ethnicity was self-identified. Hypertension was defined as systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, and/or use of antihypertensive medications. A participant was considered to have diabetes mellitus if he or she reported ever being told by a physician that he or she had diabetes or “sugar diabetes” at a time other than during pregnancy, was taking insulin or a “diabetes pill” at the time of the questionnaire, or had a fasting blood glucose level greater than 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or a nonfasting blood glucose level greater than 200 mg/dL. Smoking was classified as never, current, or former smoker. Low socioeconomic status (SES) was defined as 200% of the poverty index or lower. The use of cholesterol medication was based on the response to the question, “To lower your blood cholesterol, are you now following this advice to take prescribed medicine?”
Creatinine levels were calibrated to the Cleveland Clinic laboratory standard by subtracting 0.23 mg/dL (to convert to micromoles per liter, multiply by 88.4) from the NHANES III values, and estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine level using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.24,25 We assigned an eGFR value of 200 mL/min/1.73 m2 for all individuals with an eGFR higher than 200 mL/min/1.73m2 (n = 94) because these eGFR values were thought to be biologically implausible. The main analyses were restricted to those participants with an eGFR higher than 15 mL/min/1.73 m2. Urinary albumin to creatinine ratio was based on a spot urine sample. Both albumin to creatinine ratio and C-reactive protein values were log-transformed to achieve approximate normality for statistical analyses.
Physical activity level was based on the participant’s metabolic expenditures in the past month and categorized as low (≤3.5 METs [metabolic equivalent tasks]), moderate (3.6-14.9 METs), or high (≥15 METs) physical activity intensity.26 No information was available about whether the physical activities were performed indoors or outdoors. Use of vitamin D supplementation was coded as positive for patients who reported taking a supplement or multivitamin containing more than 0 IU of vitamin D (ergocalciferol or cholecalciferol). Those taking repletion doses of vitamin D (50 000 IU) were excluded from the analysis (n = 3).
Mortality outcomes were collected on NHANES III participants through December 31, 2000, using probabilistic matching, with up to 12 identifying data elements, to National Death Index (NDI) records and were made available in June 2005. A selected sample of death certificates was reviewed manually to validate the process. The underlying cause of death was coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)27 for deaths occurring between 1988 and 1998, and according to the International Statistical Classification of Diseases, 10th Revision (ICD-10)28 for deaths occurring in 1999 and 2000. All deaths from 1988 through 1998 coded under ICD-9-CM guidelines were recoded into comparable groups based on the ICD-10 underlying cause of death.27,28 For this analysis, CVD mortality included deaths coded as due to hypertensive disease (codes I10-I13), ischemic heart disease (codes I20-I25), arrhythmia (codes I44-I49), heart failure (code I50), cerebrovascular disease (codes I60-I69), or atherosclerosis or other diseases of the arteries (codes I70-I78). Cancer mortality included deaths coded as all malignant neoplasm deaths (codes C00-C95) including malignant neoplasms of digestive organs (codes C15-C26), respiratory and intrathoracic organs (codes C30-C39), and genital organs (codes C50-C63). A subanalysis of cancers previously linked to low 25(OH)D levels included colorectal cancer (codes C18-C21), breast cancer (code C50), and prostate cancer (code C61). Infectious deaths included deaths from infectious and parasitic diseases (codes A00-B99), meningitis (codes G00 and G03), acute rheumatic fever (codes I00-I02), endocarditis (code I33), acute upper and lower respiratory tract infections including influenza and pneumonia (codes J00-J06 and J10-J22), and urinary tract infections (code N39). External causes of mortality included deaths from accidents, falls, suicide, homicide, and complications of medical and surgical care (codes V01-Y89).
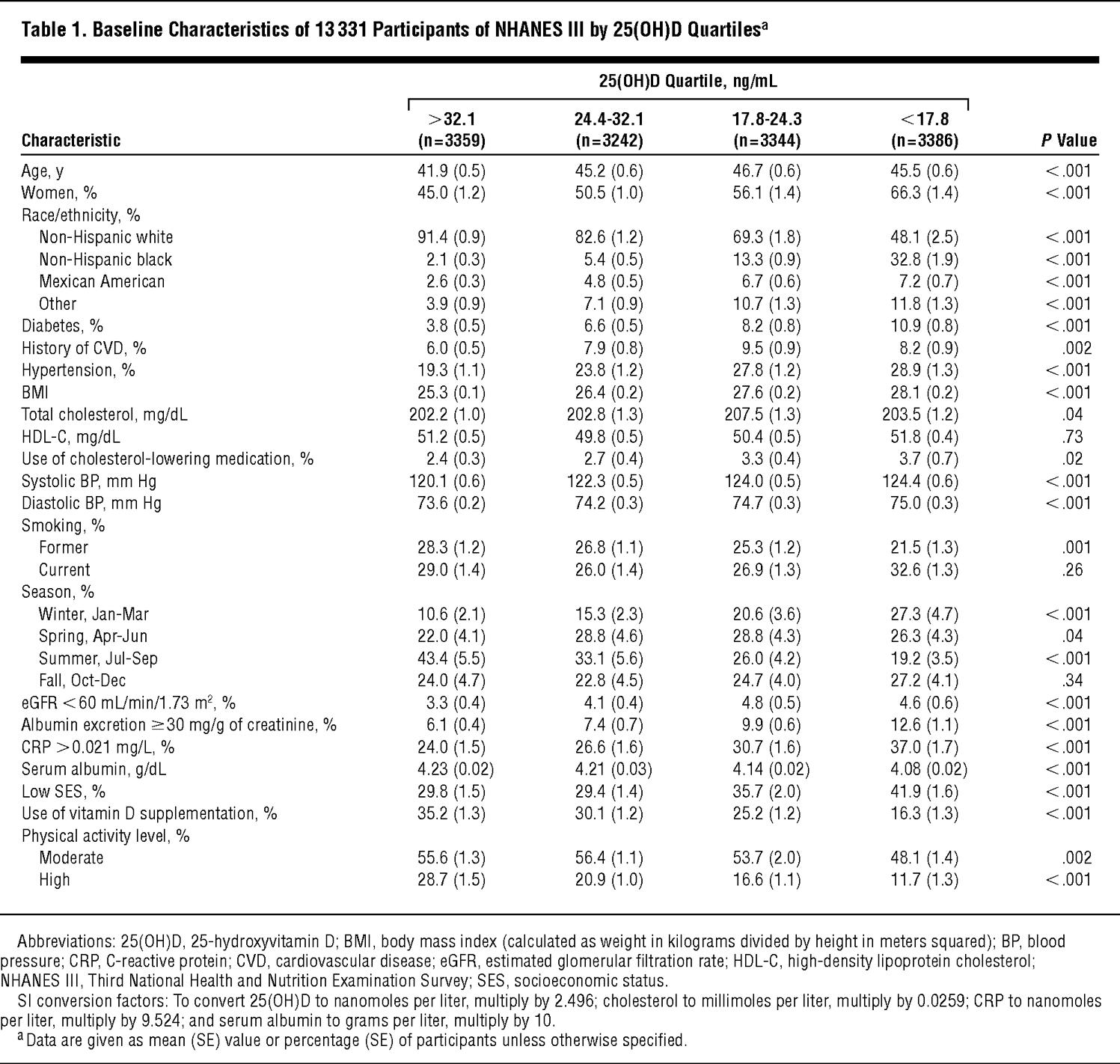
Because of the complex sampling design of NHANES III, weighted analyses using the “survey” command in Stata 9.0 (StataCorp, College Station, Texas) were used. The distributions of participant characteristics were first examined by unweighted serum 25(OH)D level quartiles (Table 1 gives the quartile cutoff values). Multivariate logistic regression analyses were used to determine independent predictors of 25(OH)D deficiency, defined as being in the lowest quartile (25[OH]D levels <17.8 ng/mL). A sensitivity analysis was performed using multivariate linear regression, with log-transformed 25(OH)D levels as the dependent variable. Power calculations using PS power (http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize) and sample size program29 revealed a detectable mortality rate ratio (MRR) of greater than 1.17 or less than 0.84 for all-cause mortality (1.25 or 0.78, respectively, for CVD mortality) assuming 80% power and an α level of .05.
Poisson regression models were used to examine the independent associations between 25(OH)D levels and cancer, CVD, other causes, and all-cause mortality. For the analyses of cause-specific mortality, participants were censored at the time of death from other causes. We explored the continuous, potentially nonlinear, relationship of mortality risk associated with 25(OH)D levels using fully adjusted restricted cubic spline models.
Xem thêm : Soplos cardíacos
Because serum 25(OH)D levels vary by season,30 all multivariate analyses were adjusted for season of examination. Inclusion in the final model was based on the variable of interest being associated with both 25(OH)D levels and mortality (P < .20) and on a priori determination of confounders of the association between 25(OH)D levels and mortality. Covariates included in the final model were age, sex, race, season, hypertension, history of CVD, diabetes mellitus, smoking, body mass index (BMI), high-density lipoprotein cholesterol, total cholesterol, the use of cholesterol-lowering medications, eGFR categories, serum albumin level, log urinary albumin to creatinine ratio, log C-reactive protein, physical activity level, vitamin D supplementation, and low SES. No pairs of covariates with correlations of 0.7 or greater were included in the models to avoid issues of colinearity. Interactions were tested by adding a product term for 25(OH)D quartile and each of the following covariates: age, sex, race, history of CVD, diabetes status, hypertension status, obesity status, physical activity level, smoking, and baseline eGFR lower than 60 mL/min/1.73 m2. Because non-Hispanic blacks have lower serum 25(OH)D levels compared with non-Hispanic whites, the highest quartile of 25(OH)D (the comparison group) only had 371 non-Hispanic blacks, compared with 2164 in the lowest quartile. Because this small comparison group may spuriously influence results, we elected to analyze non-Hispanic black-specific quartiles for the subgroup analysis (the cutoff values were >24 ng/mL [quartile 1], 18 to ≤24 ng/mL [quartile 2], 13.5 to ≤18 ng/mL, and <13.5 ng/mL [quartile 4]).
In addition, to test the robustness of the association, we performed sensitivity analyses adjusting for serum calcium and phosphate levels, which are affected by vitamin D status and are associated with mortality in patients with end-stage renal disease31 and in the general population.32 Because diabetes mellitus and/or hypertension may be in the causal pathway between low 25(OH)D levels and mortality, we developed models with and without diabetes mellitus and hypertension. Because of potential nonlinear associations between continuous variables such as age, BMI, and mortality, we also performed a sensitivity analysis in which all variables were added to the model as categorical variables (quartiles of the continuous variables). We also further tested the nonlinear association found in the spline by using the following cutoffs for 25(OH)D levels: lower than 20 ng/mL, 20 to 29 ng/mL, 30 to 39 ng/mL, 40 to 49 ng/mL, and 50 ng/mL or higher, with 30 to 39 ng/mL as the reference group. We calculated the attributable risk percentage by dividing the elevation from baseline rate (1.0) by the MRR for the lowest quartile of 25(OH)D level. We calculated the population attributable risk percentage by multiplying the attributable risk percentage by the percentage of the population who were in the lowest 25(OH)D quartile. For all analyses, P < .05 for 2-tailed tests was considered statistically significant. P values were not adjusted for multiple comparisons.
Older participants, women, and non-Hispanic blacks had lower 25(OH)D levels (Table 1 and Table 2). Across decreasing quartiles of 25(OH)D level (highest quartile to lowest), mean systolic and diastolic BP, mean BMI, and percentages of patients with diabetes, with elevated albumin to creatinine ratios, and with elevated C-reactive protein levels increased and mean serum albumin levels decreased. There were more participants with low SES and fewer participants taking vitamin D supplements and participating in high and moderate levels of physical activity in the lowest 25(OH)D quartile (<17.8 ng/mL).
In multivariate models, increasing age, female sex, nonwhite race/ethnicity (particularly non-Hispanic black race/ethnicity), diabetes, current smoking, and increasing BMI were all independently associated with an increased odds of being 25(OH)D deficient (ie, lowest quartile) (Table 3). Physical activity, vitamin D supplementation, and nonwinter season were associated with a decreased odds of deficiency. In unadjusted analysis, low SES was associated with a higher risk of deficiency (OR, 1.60; 95% CI, 1.40-1.81), which after adjustment became a lower risk of deficiency (OR, 0.73; 95% CI, 0.60-0.89). The primary confounders of this association were race and physical activity; both associated with a greater than 15% change in the point estimate. When using log-transformed 25(OH)D levels in a linear regression, all of the associations given in Table 3 remained the same, including the reversal of the low SES point estimate with adjustment.
During a median 8.7 years of follow-up (intraquartile range, 7.1-10.2 years), there were 1806 deaths, of which 777 (43%) were ascribed to CVD, 424 (23%) were ascribed to cancers, 105 (6%) were ascribed to infectious causes, and 92 (5%) were ascribed to external causes of death. Of those who died of CVD, 590 (76%) died of atherosclerotic CVD, 145 (19%) of cerebrovascular disease, and 42 (5%) of congestive heart failure. Those who died had a mean age of 66.4 years and 46% were female, 58% were hypertensive, 19% had diabetes, and 32% had prior CVD.
In unadjusted analysis, having a 25(OH)D level in the lowest quartile was associated with a 78% increased risk of all-cause mortality (Table 4). The continuous, fully adjusted association between vitamin D levels and all-cause mortality is shown graphically in Figure 1. After further adjusting for known CVD risk factors including BMI and renal function, low SES, and markers of a healthy lifestyle including physical activity and use of vitamin D supplementation, there was still a 26% higher rate of all-cause mortality for the lowest 25(OH)D quartile (MRR, 1.26; 95% CI, 1.08-1.46) compared with the highest quartile. This adjusted MRR translates to an attributable risk percentage of 20.6% and a population attributable risk percentage of 3.1% associated with the lowest 25(OH)D quartile.
For CVD mortality, the unadjusted analysis revealed that having a 25(OH)D level in the lowest quartile was associated with a 70% higher risk of mortality. This association did not remain statistically significant in the fully adjusted model, though the magnitude of the point estimate was similar to that for all-cause mortality (MRR, 1.20; 95% CI, 0.87-1.64) (Table 4). There was a nonsignificant association between lowest 25(OH)D quartile and cancer-specific mortality in an unadjusted model (MRR, 1.31; 95% CI, 0.96-1.81), which disappeared after adjustment for age, sex, race, and season (MRR, 1.05; 95% CI, 0.74-1.47). When testing only cancers previously associated with 25(OH)D levels (colorectal, breast, and prostate cancer [n = 116]), the lowest quartile had an adjusted MRR of 1.38 (95% CI, 0.49-3.93) and the 17.8- to 24.4-ng/mL quartile had an MRR of 2.05 (95% CI, 1.14-3.68).
Results did not change significantly when serum calcium and phosphate levels were entered in the fully adjusted model (MRR for lowest 25[OH]D quartile, 1.27 [95% CI, 1.10-1.47] for all-cause mortality; 1.21 [95% CI, 0.89-1.65] for CVD mortality). Likewise, the associations remained relatively unchanged in models assessing all-cause mortality not including diabetes mellitus and hypertension (Table 4) and, separately, in models not including diabetes (MRR, 1.28; 95% CI, 1.11-1.48) and not including hypertension (MRR, 1.26; 95% CI, 1.08-1.45). When continuous variables such as age were added to the model as categorical variables, the association between the lowest 25(OH)D quartile and mortality strengthened (MRR, 1.34; 95% CI, 1.15-1.57). Results also did not differ when analyzed by different 25(OH)D cutoff values. As shown in Figure 2, in women, having both low (<20 ng/mL) and high (>50 ng/mL) 25(OH)D levels was associated with an increased rate of mortality.
The lowest 25(OH)D quartile was more strongly associated with mortality among participants without a history of CVD (MRR, 1.54; 95% CI, 1.23-1.94) than in those with a history of CVD (MRR, 0.90; 95% CI, 0.66-1.22) (P value for interaction, .006) (Table 5). Stronger associations, although nonsignificant, were found for participants without hypertension (P value for interaction, .09), participants without diabetes (P value for interaction, .09), and for women (P value for interaction, .06). No interaction was observed for age, race, obesity, physical activity level, smoking, or eGFR.
In the general US population, we found that 25(OH)D deficiency (lowest quartile, <17.8 ng/mL) was associated with a 26% higher risk of all-cause mortality, independent of baseline demographics, traditional and nontraditional CVD risk factors, and measures of a healthy lifestyle. The estimated association with increased risk of CVD mortality was similar, though not statistically significant. We did not find an association with cancer mortality or other causes of death. This is the first study, to our knowledge, to explore the association between 25(OH)D levels and mortality in the general population.
Several lines of evidence suggest that vitamin D deficiency may be a risk factor for cardiovascular, cancer, and all-cause mortality. Ecological studies reveal that CVD events are higher in the winter when vitamin D levels are lower33 and that cancer survival is better if the cancer is diagnosed in the summer when vitamin D levels are higher.34 In addition, observational data show that the use of activated vitamin D therapy in patients with end-stage renal disease is associated with decreased mortality.6-10 Vitamin D receptor knockout mice experience cardiac myocyte hypertrophy.2 Activated vitamin D has antiproliferative properties.5 In mice, vitamin D is an inhibitor of the renin-angiotensin system.1 Other inhibitors of the renin-angiotensin system, such as angiotensin-converting enzyme inhibitors, reduce mortality and morbidity in multiple disease states.35
Low 25(OH)D levels are also associated with hypertension, diabetes mellitus, insulin resistance, and an elevated BMI, all of which are risk factors for CVD and all-cause mortality. Low 25(OH)D levels,12 but not vitamin D intake,36 are associated with incident hypertension. There are 2 small clinical trials that suggest that vitamin D supplementation37,38 reduced systolic BP. The association of 25(OH)D deficiency with obesity,39,40 glucose intolerance,15,16,41 and the metabolic syndrome42 is another potential mechanism for increased CVD risk. We found that diabetes mellitus and increased BMI were independently associated with increased odds of being 25(OH)D deficient. We adjusted for hypertension, diabetes mellitus, and BMI in our analyses and still found a statistically significant association with all-cause mortality. Analyses with and without hypertension and diabetes mellitus in the model did not reveal a difference in the risk estimates associated with low 25(OH)D levels. However, this analysis did not account for incident diabetes and hypertension developing after the NHANES survey period.
The association of low 25(OH)D levels with mortality was strongest in those without CVD, without hypertension, and without diabetes mellitus, arguing against low vitamin D levels being only a marker of poor general health. If diabetes mellitus and hypertension are in the causal pathway between low 25(OH)D levels and mortality (ie, low 25[OH]D leads to hypertension, which leads to higher mortality), it follows that the effect is more pronounced in those without preexisting diabetes mellitus, hypertension, and CVD. The fact that the associations were stronger in those without CVD at baseline suggests that if a causal relationship exists, 25(OH)D deficiency may play a role before CVD is established.
It is unclear why the association between 25(OH)D levels and mortality was more pronounced among women. It may be that there is a hormone interaction between estrogen levels and 25(OH)D. Compared with men, women tend to develop atherosclerosis later in life, suggesting that, as in the subgroup without CVD, low 25(OH)D levels play a role in the development of atherosclerosis. This area deserves further research and may help reveal the mechanisms behind the associations seen, if in fact they are causal.
Xem thêm : Is Stomach Wall Thickening Serious?
A recently published randomized clinical trial of calcium and vitamin D3 supplementation (400 IU/d) in generally healthy postmenopausal women did not show a lower risk of cardiovascular events in those randomized to vitamin D and calcium supplementation.43 However, a meta-analysis of 18 randomized clinical trials showed that participants randomized to vitamin D supplementation experienced fewer deaths compared with those randomized to placebo.18 In that analysis, as in ours, they were not able to establish the specific cause of death responsible for the lower mortality.
Several authors have commented that the optimal levels of 25(OH)D should be greater than 30 ng/mL.19,20 In our observational study, we found that there was a lower risk of mortality at levels of 30 to 49 ng/mL, but that at levels greater than 50 ng/mL there was again a higher risk of mortality in women. This is similar to findings about antioxidant vitamins and vitamin E, which show that too much may be harmful.44,45
Several associations between vitamin D deficiency and clinical factors need further explanation. In adjusted analysis, low SES was associated with a lower risk of being 25(OH)D deficient. Most of the confounding was due to the very strong association between non-Hispanic black race and low 25(OH)D status. The mechanism for the protective effect of low SES is unknown. However, one may conjecture that participants with low SES may spend more time outdoors and therefore have more sun exposure.
Our study is limited in that it is an observational study, and therefore causality cannot be inferred. We adjusted for all known traditional CVD risk factors and nontraditional risk factors, including markers of a healthy lifestyle, such as the use of vitamin D supplementation. Residual confounding, however, may still exist. Specifically, the excess mortality observed in the lowest quartile may be a reflection of the participant’s overall poor condition or an unmeasured confounder, which we could not fully adjust for in our analysis. We were not able to distinguish the specific causes of mortality that accounted for the elevated all-cause mortality risk associated with 25(OH)D deficiency in our population, perhaps owing to limitations of power or to the use of potentially imprecise death certificate data. Northern states were only sampled in the summer in NHANES III, and therefore the full extent of 25(OH)D deficiency in the population is probably underestimated. This probably attenuates the association seen in our results. Finally, one must view the subgroup analyses with caution because of issues of multiple comparisons.
In conclusion, the lowest 25(OH)D quartile (<17.8 ng/mL) is associated with a higher risk of all-cause mortality in the general US population. Further observational studies are needed to confirm these findings and establish the mechanisms underlying these observations. If confirmed, randomized clinical trials will be needed to determine whether vitamin D supplementation at higher doses could have any potential benefit in reducing future mortality risk in those with 25(OH)D deficiency.
Correspondence: Michal L. Melamed, MD, MHS, Division of Nephrology, Department of Medicine, Albert Einstein College of Medicine, 1300 Morris Park Ave, Ullmann 615, Bronx, NY 10461 (mmelamed@aecom.yu.edu).
Accepted for Publication: January 15, 2008.
Author Contributions: Drs Melamed and Michos had full access to all the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and contributed equally to this work. Study concept and design: Melamed and Michos. Acquisition of data: Melamed and Michos. Analysis and interpretation of data: Melamed, Michos, Post, and Astor. Drafting of the manuscript: Melamed and Michos. Critical revision of the manuscript for important intellectual content: Melamed, Michos, Post, and Astor. Statistical analysis: Melamed, Michos, and Astor. Obtained funding: Melamed. Study supervision: Michos, Post, and Astor.
Financial Disclosure: Dr Michos has received consulting fees from Abbott Pharmaceuticals.
Funding/Support: Dr Melamed and this analysis were supported by grant F32-DK069017 and K23-DK078774 and Dr Michos was supported by grant T32-HL007024 from the National Institutes of Health. Dr Michos is also supported by the PJ Schafer Cardiovascular Research Fund. Dr Post is supported, in part, by the Paul Beeson Physician Faculty Scholars in Aging Program.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation, review, or approval of the manuscript.
Disclaimer: All analyses, interpretations, and conclusions are made by the authors and do not represent views of the National Center for Health Statistics (NCHS).
Additional Information: The NCHS is the source of the data used in this analysis.
Additional Contributions: Christopher Rogers, PhD, of the NCHS aided in the analysis.
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on November 22, 2024 6:23 am