Abstract
Obesity is a multifactorial chronic disease for which treatment remains challenging. The Centers for Disease Control and Prevention estimate US adult obesity [defined as body mass index (BMI) ≥ 30 kg/m2] at 41.9%, including 9.2% for severe obesity (defined as BMI ≥40 kg/m2) [1]. Treating obesity is critical for reducing the risk of multiple complications and comorbidities including type 2 diabetes mellitus, dyslipidemia, hypertension, cardiovascular events, nonalcoholic fatty liver disease, and obstructive sleep apnea, among others [2].
Lifestyle modification therapy, including dietary changes and increased physical activity supported by behavior modification strategies, remains the cornerstone treatment for obesity [2]. That said, this approach commonly fails to result in clinically significant and durable weight loss [2]. Therefore, adjunct therapy is often needed. While metabolic/bariatric surgery (MBS) provides on average the most effective and durable BMI reduction with a mean weight loss of ≥30% and significant reductions in obesity-related comorbidities [3], such procedures are invasive, carry risk, and may not be attractive or available to many individuals.
Bạn đang xem: Weight Loss From Combination Anti-Obesity Medication Regimens Can Approach that Achieved From Bariatric Surgery
Anti-obesity medications (AOMs) are promising adjuncts to lifestyle modification therapy as their addition to treatment regimens result in greater weight reduction, improved adherence to lifestyle modification therapy, and greater weight loss maintenance compared to lifestyle modification therapy alone [2]. Current Food and Drug Administration AOMs approved for long-term use in adults include orlistat, topiramate/phentermine, naltrexone/bupropion, liraglutide 3.0 mg daily, semaglutide 2.4 mg weekly, and setmelanotide (for specific genetic etiologies). Additionally, other medications are used off-label including phentermine (when used for ≥3 months), topiramate, and lisdexamfetamine, among others [4].
When an individual does not respond to lifestyle modification therapy combined with a single AOM, combination AOM therapy should be considered, consistent with recommendations for other chronic diseases including type 2 diabetes mellitus and hypertension. Indeed, several combination AOMs currently exist (ie, phentermine/topiramate, bupropion/naltrexone), others are being studied [ie, tirzepatide, a glucagon-like peptide 1 (GLP-1) receptor agonist/glucose-dependent insulinotropic polypeptide (GIP) dual agonist], and others are used in clinical practice (ie, combining GLP-1 receptor agonists with phentermine). Overall, studies involving many of these combination AOM regimens are lacking. Given the promise of combination AOM regimens including GLP-1 receptor agonists, we present a case illustrating the potential significant effect of this approach showing results mirroring those expected from MBS.
Case Presentation
A 23-year-old male presented to a weight management program with a weight of 643 pounds (292 kg) and BMI of 84.3 kg/m2. On exam, obesity was mixed central and peripheral. Factors contributing to his weight status included a genetic predisposition (significant family history of obesity in immediate family members including his mother and sister), strong degrees of hunger, food cravings, binge-eating tendencies, and depression. He also had evidence of prediabetes with a hemoglobin A1c of 6.4% and concerns for obstructive sleep apnea.
He was previously followed in a pediatric weight management clinic where he originally presented at 17 years old with a weight of 364 pounds (165 kg; BMI 48.5 kg/m2). At that time, he was initially started on topiramate 75 mg daily, and phentermine 15 mg daily was added shortly thereafter. He continued on these medications for around 6 months during which time he lost approximately 25 pounds (11.3 kg). He was then lost to follow-up several times over the next few years and therefore was not consistently on AOMs. He was briefly restarted on phentermine and topiramate; however, he was on these for only a few weeks at a time. Each time he represented for care, his weight increased approximately 50 to 75 pounds (23-34 kg).
Upon re-establishing care in the adult weight management clinic, he was started on multiple medications including phentermine 15 mg, topiramate 75 mg, and metformin 500 mg daily. With the COVID-19 pandemic, he was seen virtually via telemedicine over the next year, and weight and BMI therefore could not be obtained. During that time, phentermine was increased to 18.75 mg daily, topiramate to 50 mg twice a day, and metformin to 500 mg twice a day. Given a history of depression, he also started bupropion extended release 150 mg daily by his primary care provider, which was eventually increased to 300 mg daily.
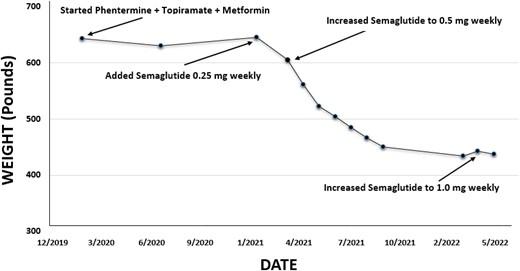
When he was ultimately able to return to weight management clinic in person, his weight and BMI remained largely unchanged. Therefore, in addition to continuing phentermine, topiramate, metformin, and bupropion, he was started on semaglutide 0.25 mg weekly (a GLP-1 receptor agonist), along with a lower calorie diet and regularly scheduled registered dietician visits. He subsequently reported a significant reduction in hunger, and his BMI began to decrease. One month later, semaglutide was increased to 0.5 mg weekly. After just over a year on this combination regimen, he lost 209 pounds (95 kg) and his BMI decreased to 66.5 kg/m2 (see Fig. 1). Additionally, his hemoglobin A1c normalized to 5.0% and snoring improved.
Xem thêm : Herbal Safety
After approximately 14 months on semaglutide 0.5 mg weekly (in addition to phentermine, topiramate, metformin, and bupropion), given a plateau in weight loss, semaglutide was increased to 1.0 mg weekly. The first month after this increase, he lost an additional 6 pounds (2.7 kg); however, after 3 months he had gained an additional 11 pounds (5 kg), suggesting overall stability on this AOM regimen rather than further BMI reduction.
Discussion
As highlighted in this case, combination AOMs including GLP-1 receptor agonists may substantially benefit individuals with refractory obesity. Current recommendations are to use AOM monotherapy or Food and Drug Administration-approved combination medications [2]. However, weight loss achieved with these regimens may be either modest or suboptimal [2], and therefore combination therapies involving multiple different medications may be required, as was the case here [5]. This patient was initially started on phentermine, topiramate, metformin, and bupropion after re-establishing care in a weight management clinic, on which his BMI remained unchanged. This suggested he was experiencing some benefit as, during the time when he was not on any medications, his BMI continued to substantially increase.
That said, when a GLP-1 receptor agonist (semaglutide) was added, his BMI began to significantly decrease. GLP-1 receptor agonists appear to work via multiple pathways to help induce BMI reduction. These include delayed gastric emptying, which can improve satiety; central nervous system effects leading to decreased hunger, appetite, and food cravings; lower preference for energy-dense foods; and improvements in eating control [2]. After semaglutide was added to his AOM regimen, he experienced a further reduction in hunger and was able to better adhere to a lower calorie diet, possibly due to improved satiety [2]. In this case, it is unclear as to whether initially starting semaglutide as monotherapy would have led to the same BMI reduction compared to using semaglutide as an adjunct to other medications. That said, the BMI reduction achieved after starting semaglutide 0.5 mg weekly far exceeded the average described in the SUSTAIN trials (among adults with type 2 diabetes), as well as mean weight loss achieved on higher doses of semaglutide (eg, 2.4 mg weekly) among adults without obesity [6]. This, combined with BMI stability achieved on other medications, suggests a possible synergy in response between these medications.
While outcomes for metabolic/bariatric in adults vary, studies have shown a mean 1 year weight loss of 31.5% for those undergoing Roux-en-Y gastric bypass and 29.5% for those undergoing laparoscopic sleeve gastrectomy [3]. While true to date that, on average, MBS has provided more significant and durable weight loss compared to AOMs, many patients unfortunately experience significant weight gain following MBS (often requiring AOMs in the post-surgical setting), and more recently developed AOMs are, on average, far more effective compared to those previously available [6-8]. For example, in randomized controlled trials, compared to a mean weight reduction of 10.9% after 56 weeks with phentermine/topiramate, mean body weight with semaglutide 2.4 mg weekly was 14.9% after 68 weeks and 20.9% with tirzepatide after 72 weeks [6-8].
The weight loss this patient achieved using combination AOMs including a GLP-1 receptor agonist was 32.5%, mirroring average weight loss experienced following metabolic/bariatric surgery [3]. In this case, it may have been that addition of a GLP-1 receptor agonist to an ongoing combination of other medications used in weight management (phentermine, topiramate, metformin, and bupropion) provided a synergistic effect. We hypothesize that this resulted from a combination of medications targeting multiple eating behavior pathways simultaneously, including appetite, satiety, food craving, and binge-eating tendencies (see Table 1). Indeed, as body weight is regulated by a complex physiological network, combination AOM regimens may achieve weight loss by targeting multiple pathways simultaneously. It is likely that most patients will require AOM use long term; however, studies showing the impact of discontinuation are currently lacking.
To date, few studies have examined weight loss benefits from the addition of GLP-1 receptor agonists (in this case semaglutide) to ongoing medication regimens used for weight management, with mixed results [9, 10]. For example, Simonds et al showed an additive effect when liraglutide (a GLP-1-receptor agonist) was added to phentermine in mice [9]. However, in a randomized placebo-controlled pilot study in adults with obesity on liraglutide for 1 year (average 12% weight loss on liraglutide), the addition of phentermine did not provide meaningful additional weight loss compared to placebo [10]. That said, this study included only 45 participants, and, in this case, the GLP-1 receptor agonist was initiated prior to phentermine, which was opposite to our case. Further studies on combination AOM regimens are therefore needed, including those involving newer combination medications such as tirzepatide [5, 8].
In summary, we report the case of a 23-year-old male with severe obesity (initial BMI 84.3 kg/m2) and obesity-related complications including prediabetes who experienced weight stabilization, as opposed to continued weight increase, on a combination medication regimen including phentermine, topiramate, metformin, and bupropion. After a GLP-1 receptor agonist (semaglutide) was added, he experienced a substantial BMI decrease with a 32.5% reduction in total body weight. This result mirrors what is generally expected following MBS and suggests that combination AOM regimens including GLP-1-receptor agonists may lead to substantial weight loss benefits. There is a need for further studies involving combination AOM regimens that include GLP-1 receptor agonists for obesity management.
Learning Points
-
While lifestyle modification is the cornerstone obesity treatment, this commonly fails to result in clinically significant and durable weight loss and, therefore, adjunct anti-obesity medications are often necessary.
-
Xem thêm : U.S. Food and Drug Administration
In cases where monotherapy with a single anti-obesity medication results in either weight stabilization or only modest weight reduction, combination regimens can be highly effective as such regimens target multiple eating behavior pathways simultaneously.
-
Combination anti-obesity medication regimens including glucagon-like peptide-1 receptor agonists can be highly effective and may result in weight loss approximating that achieved through metabolic/bariatric surgery
-
Further research is needed to evaluate the effectiveness of combination anti-obesity medication regimens, especially those including glucagon-like peptide-1 receptor agonists.
Contributors
All authors made individual contributions to authorship. P.N.P.: wrote the first draft of the manuscript, created tables and figures, and made critical revisions to the manuscript for intellectual content. C.K.F., E.M.B.: made critical revisions to the final manuscript and tables/figures for intellectual content. E.M.B.: was involved in the management of this patient and made critical revisions to the manuscript and tables/figures for intellectual content. All authors reviewed and approved the final draft.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK125668 and K23DK129721. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
C.K.F. and E.M.B. are site principal investigators and site coinvestigators for Novo Nordisk. P.N.P. and M.O.B. have no financial disclosures. All authors have no relevant conflicts of interest to disclose regarding the content of this manuscript.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on December 5, 2024 6:19 am