Importance Mitotic rate is now recognized as having independent prognostic significance in melanoma survival. However, its clinicopathologic associations have not been the focus of any previous study.
Objective To identify a set of patient and tumor characteristics associated with high-mitotic-rate melanoma with the aim of facilitating the earlier detection of aggressive primary invasive melanoma.
Bạn đang xem: Characteristics and Associations of High-Mitotic-Rate Melanoma
Design, Setting, and Participants Cross-sectional study of patients from a multidisciplinary melanoma clinic based in a public hospital. A total of 2397 cases from January 2006 to December 2011 were reviewed by the Victorian Melanoma Service, and 1441 patients with 1500 primary invasive melanomas were included in the study.
Main Outcomes and Measures Mitotic rate was measured as number of mitoses per mm2 and analyzed as ordered categories (0, <1, 1 and <2, 2, 3-4, 5-9, and ≥10) according to patient demographics, phenotypic markers, historical data, tumor presentation, and histopathologic features.
Results Melanomas with higher mitotic rates were more likely to occur in men (odds ratio [OR], 1.5; 95% CI, 1.3-1.8), patients 70 years or older (OR, 2.1; 95% CI, 1.7-2.8), and those with a history of solar keratosis (OR, 1.3; 95% CI, 1.1-1.6). These melanomas occurred more frequently on the head and neck (OR, 1.4; 95% CI, 1.0-1.9) and presented more often as amelanotic (OR, 1.9; 95% CI, 1.4-2.5) and rapidly growing (≥2 mm/mo) lesions (OR, 12.5; 95% CI, 8.4-18.5). An association was seen with the nodular melanoma subtype (vs superficial spreading [reference]) (OR, 2.5; 95% CI, 1.8-3.4), greater tumor thickness (vs ≤1 mm [reference]) (>1-4 mm: OR, 4.5; 95% CI, 3.2-6.1; >4 mm: OR, 12.6; 95% CI, 7.5-21.1), and ulceration (OR, 2.0; 95% CI, 1.5-2.7). These histopathologic features, along with amelanosis and rate of growth, remained as significant associations with high mitotic rate in the overall multivariate analysis.
Conclusions and Relevance High-mitotic-rate primary cutaneous melanoma is associated with aggressive histologic features and atypical clinical presentation. It has a predilection for the head and neck region and is more likely to be seen in elderly men with a history of cumulative solar damage who present clinically with rapidly developing disease.
Mitotic rate, a quantifiable marker of tumor cellular proliferation, has been closely correlated with survival, some studies demonstrating independent prognostic significance.1-11 For thin tumors, 1 mm or thinner, the mitotic rate is now a criterion alongside ulceration for defining T1b melanoma.4
Previous studies of aggressive melanoma have focused on the characteristics and associations of thick melanoma. Thick melanomas have been shown to have a strong association with the nodular subtype,12-17 to grow rapidly, and to occur more frequently in elderly men and in individuals with fewer nevi and fewer freckles.18,19 However, the existing literature has scarce data regarding the clinical presentation and associations of high-mitotic-rate melanoma to assist in identifying those at risk for poor prognosis. The aim of this study was to delineate the clinical, phenotypic, and histologic associations of high-mitotic-rate melanoma.
Institutional ethics approval was obtained from the Alfred Hospital for a retrospective review of all patients seen at the Victorian Melanoma Service (VMS) from January 2006 to December 2011. Patient informed consent was waived. All patients whose primary invasive melanoma was histologically reviewed and assessed for mitotic rate at the VMS were included in the study. Two expert dermatopathologists independently reviewed the original histopathology sections for each case.
The following data were collected prospectively for each subject: demographic information (age, sex, ethnicity), phenotypic markers (eye color, hair color, skin prototype), historical features (number of blistering sunburns, previous melanoma, solar keratosis, family history of a first-degree relative with melanoma), and tumor presentation (amelanosis, body site, and time from the initial observation of the lesion to confirmed diagnosis). Clinical examination of all patients undertaken by a dermatology resident provided count of total nevi, clinically determined dysplastic nevi, freckles, and solar lenities. The count of total number of melanocytic nevi was grouped (<20, 20-50, >50-100, >100-200, >200-500, and >500) based on previously observed risk thresholds for melanoma.20,21 Dysplastic nevi were counted exactly, and freckles and solar lentigines were recorded as few, moderate, or many. For the purpose of this study, tumor site was classified as head and neck, trunk, upper extremities, and lower extremities. We used a previously described historical estimation of rate of growth of a tumor that was derived by dividing its Breslow thickness in millimeters by the time, in months, from the initial observation of the change in the lesion to histologic diagnosis.19,22 Amelanotic tumors were those that appeared to the patient to be without pigmentation.
Mitotic rate was determined histologically by first examining the entire tumor looking for mitotic figures. The area with the greatest density of mitotic activity was used as the focus, and the number of mitoses in a surrounding area of 1 mm2 was counted. Mitotic rate was reported by the dermatopathologist either as a discrete integer or as less than 1 and less than 2 per mm2.
Tumor type was classified histologically as superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, acral lentiginous melanoma, or desmoplastic melanoma. Less common tumor types were grouped as other. Other histologic features assessed were Breslow thickness, ulceration, Clark level of invasion, lymphovascular invasion, microsatellitosis, tumor infiltrating lymphocytes, regression, neurotropism, and preexisting nevus.
Mitotic rate followed a skewed distribution and was categorized for analysis (0, <1, 1 and <2, 2, 3-4, 5-9, and ≥10 mitoses/mm2). These cutoffs were chosen to ensure reasonable numbers of patients in each category. The association of mitotic rate with each of the clinical and histopathologic parameters was summarized with nonparametric Spearman rank correlation coefficients. Ordinal logistic regression was used to examine univariate and multivariate associations between mitotic rate and other variables. Odds ratios (ORs) from this analysis can be interpreted as increased odds of a higher category of mitotic rate regardless of where the scale is dichotomized. This interpretation relies on the assumption of proportional odds, which was tested for the final multivariate model. Nonlinearity of the association between mitotic rate and thickness was explored using fractional polynomials. Statistical analyses were performed using IBM SPSS Statistics version 19.0 (IBM Corporation) and Stata version 13 (StataCorp LP) statistical software packages. P < .05 was considered statistically significant.
A total of 2397 patients presented to the VMS during the study period, and 1441 of these, diagnosed as having 1500 primary melanomas, were included in the analysis. Of the 1500 melanomas, 813 (54%) occurred in men (mean age, 60 years), and 687 occurred in women (mean age, 55 years).
Xem thêm : A Simple 3-Step Routine To Take Control of Acne
The mitotic rate ranged from 0 to 75 mitoses/mm2 (eTable in the Supplement): 41.7% of the melanomas had a mitotic rate of less than 1/mm2; 28.8% had a mitotic rate of between 1 and 2 mitoses/mm2, and the mitotic rate of remaining 29.5% of melanomas was 3 or more mitoses/mm2. The mean (SD) mitotic rate was 2.8 (4.9) mitoses/mm2 (interquartile range, 2.5/mm2).
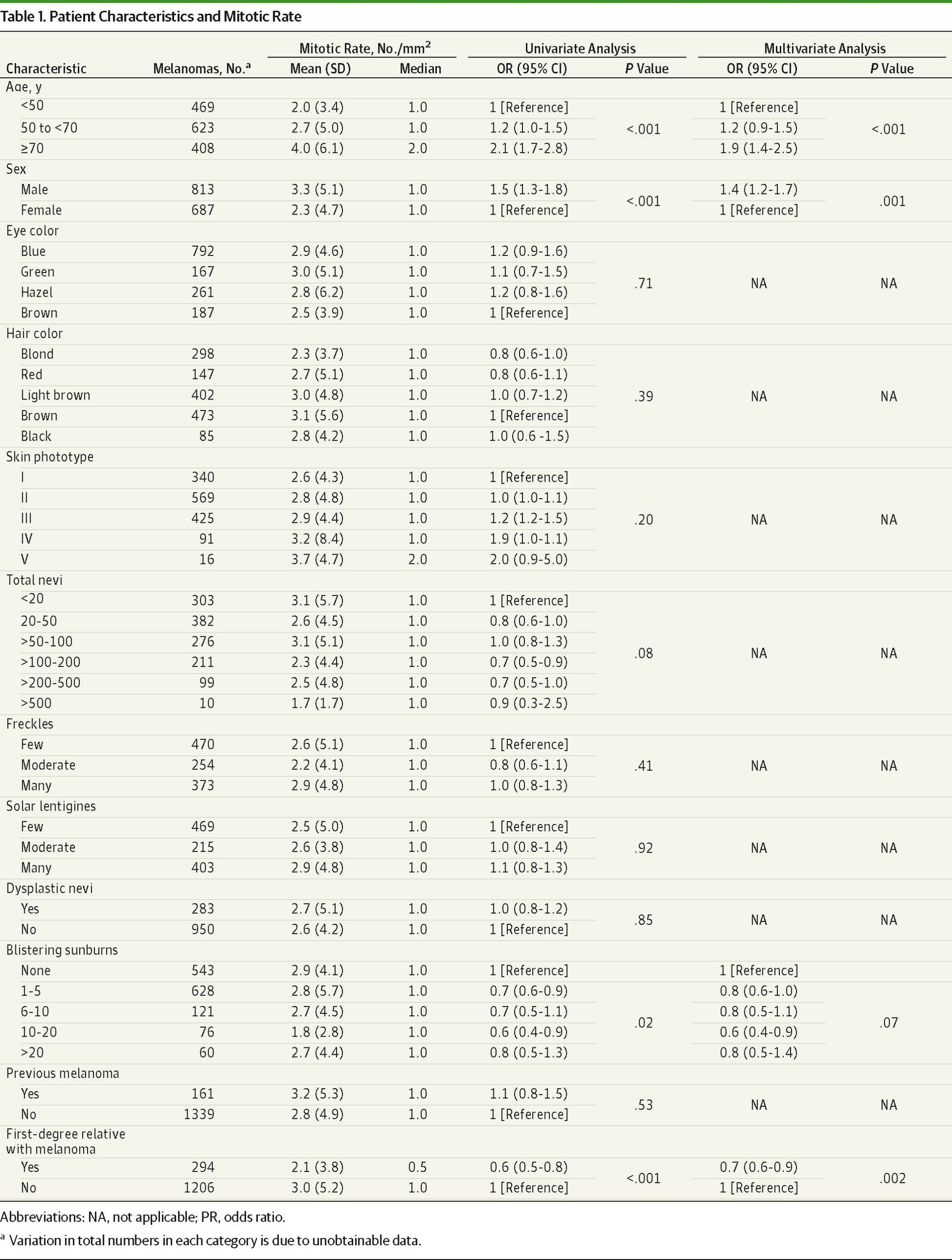
Univariate analysis demonstrated strong associations between older age (≥70 years) (OR, 2.1; 95% CI, 1.7-2.8), male (OR, 1.5; 95% CI, 1.3-1.8), and increasing mitotic rate (Table 1). Quiz Ref IDThe ratio of men to women in the highest mitotic rate group (≥10/mm2) was 2.2:1, and 45% of these patients were 70 years or older. The presence of solar keratosis was also associated with higher mitotic rate (OR, 1.3; 95% CI, 1.1-1.6). Conversely, a history of blistering sunburns and a clinically significant family history of melanoma were associated with lower mitotic activity (P = .02 and P < .001, respectively). Age 70 years or older (OR, 1.9; P < .001), male sex (OR, 1.4; P = .001), and family history (OR, 0.7; P = .002) remained as significant associations with mitotic rate on multivariate analyses of patient characteristics (Table 1).
Quiz Ref IDUnivariate analysis revealed that head and neck location (P = .005), amelanosis (P < .001), and increasing rate of growth (P < .001) had strong associations with high mitotic rate. Half (50%) of melanomas with a mitotic rate of 5 mitoses/mm2 or higher were amelanotic. Initial detection by the patient rather than the physician or others was associated with higher mitotic rate (P < .001) and remained as an independent association in the multivariate analysis (P = .007), along with amelanosis and rate of growth (P < .001 for both) (Table 2).
By univariate analysis, all histopathologic factors except tumor-infiltrating lymphocytes and desmoplasia were significantly associated with mitotic rate. Exploratory analysis indicated that the relationship between thickness and mitotic rate (eFigure 1 in the Supplement) was best characterized by an exponential increase in mitotic rate with increasing thickness, and this relationship was adequately captured by the 3 categories of tumor thickness detailed in Table 3. Quiz Ref IDPatients in the higher mitotic rate groups had thicker melanomas with greater proportions being ulcerated. In cases with a mitotic rate of 10 mitoses/mm2 or higher, over half (53.8%) were ulcerated, in contrast with only 6% in the 0 mitoses/mm2 group (Table 4). Nodular melanoma was associated with a higher mitotic rate, with a ratio of 2.3:1 compared with superficial spreading melanoma (reference) in the 10 mitoses/mm2 or higher group. Superficial spreading melanoma accounted for the majority of the low-mitotic-rate melanomas (73% in the 0/mm2 category) (eFigure 2 in the Supplement). Similarly, features of regression (OR, 0.4; 95% CI, 0.4-0.6; P < .001) and the presence of preexisting dysplastic nevi (OR, 0.2; 95% CI, 0.1-0.3; P < .001) were associated with lower mitotic activity.
Nodular tumor type, thickness, ulceration, and Clark level of invasion remained as significant predictors of high mitotic rate in the multivariate analyses of tumor and histopathologic factors. An inverse correlation remained with preexisting dysplastic nevus (Table 3).
The independent associations of mitotic rate from the 3 multivariate models were entered into a final multivariate ordinal logistic regression model. This included both patient and tumor characteristics (age, sex, first suspected by whom, amelanosis, rate of growth) and histopathologic parameters (tumor type, thickness, ulceration, Clark level of invasion, and preexisting nevus).
Nodular tumor type (OR, 2.3; 95% CI, 1.6-3.3; P < .001), ulceration (OR, 2.0; 95% CI, 1.4-2.8; P < .001), Clark level 5 invasion (OR, 5.9; 95% CI, 3.0-11.7; P < .001), and thickness greater than 4 mm (OR, 8.8; 95% CI, 4.9-15.9; P < .001) retained strong positive associations with mitotic rate, while an inverse relationship persisted with preexisting dysplastic nevus (OR, 0.4; 95% CI, 0.2-0.6; P = .001). Rate of growth (P = .01) and amelanosis (P = .03) independently correlated with higher mitotic rate, although to a lesser extent than the histopathologic variables. The person by whom malignant growth was first suspected (P = .03) also remained a significant association. The age and sex associations that were observed in earlier analyses did not appear strong in this analysis, and so their relationships with higher mitotic rate could be explained by other parameters in the analysis (Table 4).
The proportional odds assumption was rejected for the model overall; however, the large sample size translated to high power for this proportional odds test. Tests of the same assumption in the univariate analyses indicated that it was satisfied for most variables, with the 2 exceptions of thickness and tumor level, which may have more complex associations with mitotic rate than those described by the multivariate ORs. A univariate model with the logarithm-transformed values of thickness captured the exponential relationship, as identified in eFigure 1 in Supplement, but surprisingly also failed the proportional odds test, although this could be explained by evidence of a threshold at the first category of mitotic rate, that is, at the count of 0. This model with log-thickness passed the proportional odds test when restricted to melanomas with mitotic rate greater than 0.
Mitotic rate, a quantifiable measure of tumor growth, has been shown to correlate with melanoma survival.4,5 Disease-free survival has been found to decline with increasing mitotic rate, with in-transit, nodal, and distant recurrences occurring more commonly in patients with high-mitotic-rate melanoma (≥5 mitoses/mm2).23 In conjunction with Breslow thickness and ulceration, mitotic rate is a criterion in the current AJCC staging system for localized melanoma.5 However, while the presence of many mitotic figures has been recognized as a poor prognostic feature, the clinicopathologic characteristics of patients with high-mitotic-rate melanoma have yet to be formally elucidated.
This study has identified that patients with higher mitotic rate tumors were more likely to be older, to be male, to have a history of significant solar field damage, and to present with rapidly growing primary melanoma that was more likely to be located on the head and neck. Melanomas with very high mitotic activity (≥10 mitoses/mm2) were predominantly thick and ulcerated nodular tumor subtypes. Quiz Ref IDConversely, the superficial spreading melanoma subtype, features of regression, and the presence of preexisting nevi were found to be characteristic of lesions with sparse mitotic activity. These melanomas were significantly thinner than their more mitotically active counterparts and tended to occur more commonly in patients with previous blistering sunburns and a family history of a first-degree relative with melanoma. This accords with previous observations of associations between these historical factors and thin tumors.24,25
Studies investigating patients at the greatest risk of mortality from primary invasive melanoma have focused mainly on those presenting with thick lesions. There is a recognized association between tumor thickness, male sex, and older age.13,14,18 In a retrospective survey of 1124 patients, Hersey et al13 found that 68% of patients with thick melanoma (≥3 mm) were men, and 75% were older than 70 years. The head and neck region was the most common site for thick melanoma,13 although Hanrahan et al17 later observed that tumor site was not related to thickness when nodular tumor type was taken into account.
As a phenotypic marker of cumulative sun damage, solar keratosis is considered a risk factor for melanoma,26,27 and its association with melanoma occurring on the head and neck and in men has been recognized.18,27 In this study, the presence of solar keratosis was shown to be associated with high mitotic rate, although this relationship was lost when age and sex were factored into the analysis. Nodular melanoma, which demonstrated higher mitotic activity than any other tumor type in the current study, has been more strongly linked to solar keratosis than superficial spreading melanoma.28
Quiz Ref IDDespite the likelihood of being amelanotic, high mitotic rate melanomas were most commonly first discovered by patients themselves. For instance, 62.1% of melanomas with a mitotic rate of 5/mm2 or higher were first detected by patients themselves. This is possibly a result of their rapid growth rate, which, as a clinical measure of tumor proliferation, has been reported previously to closely correlate with mitotic rate.19 The same study also described an association between rapid tumor growth and atypical tumor morphology, including amelanosis, symmetry, elevation, and border regularity,19 which are recognized features of nodular melanoma.29 While tumor subtype was chosen as a histologic variable for the purposes of this study, it is important to recognize that each tumor subtype has characteristic morphologic features that are helpful in their clinical detection. The presenting features of nodular melanoma may pose a challenge in this regard owing to its symmetrically elevated, often amelanotic appearance that does not fit the classic “ugly duckling” radial growth phase melanoma as defined by the ABCDE rule (asymmetry, border irregularity, color variegation, diameter >6 mm, and evolving) (Figure).30
The previous observation that thick and ulcerated melanomas are mitotically active7,31 was consistent with the current findings. However, it must be noted that desmoplastic melanoma, which was found to be comparable in thickness to nodular melanoma, exhibited low mitotic activity. This may serve to explain, to a degree, the distinct clinical behavior suggested for this rare melanoma subtype that is characterized by its propensity for local recurrence but a lower incidence of distant spread relative to other forms of cutaneous melanoma.32,33
Xem thêm : Neurotropas 25000 – 1 Ampolla
To our knowledge, this is the first formal description of the clinicopathologic associations of high-mitotic-rate melanoma. The results from this single-center study merit replication elsewhere to confirm generalizability and to further explore the potential implications for detection and treatment of at-risk patients, who in this study were found to have a distinct phenotypic and historical profile. Mitotically active melanomas were more often seen in older men with chronic solar field damage. These tumors have a predilection for the head and neck and can present with nodular structure and amelanosis. Such atypical clinical features may pose a challenge to timely detection; thus a high index of suspicion is warranted when the patient reports a history of morphologic change and rapid growth.
Corresponding Author: Sarah Shen, MBBS, BMedSci, Alfred Hospital, 55 Commercial Road, Prahran, 3181 Victoria, Australia (sshenwq@gmail.com).
Accepted for Publication: January 31, 2014.
Published Online: August 20, 2014. doi:10.1001/jamadermatol.2014.635.
Author Contributions: Drs Shen and Kelly had full access of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shen, McLean, Kelly.
Acquisition, analysis, or interpretation of data: Shen, Wolfe, McLean, Haskett, Kelly.
Drafting of the manuscript: Shen, Kelly.
Critical revision of the manuscript for important intellectual content: Shen, Wolfe, McLean, Haskett, Kelly.
Statistical analysis: Shen, Wolfe.
Obtained funding: Kelly.
Administrative, technical, or material support: Shen, McLean, Kelly.
Study supervision: Wolfe, McLean, Haskett, Kelly.
Conflict of Interest Disclosures: None reported.
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on November 21, 2024 12:14 pm