Alcohol
Overview
In the healthcare setting, “alcohol” refers to two water-soluble chemical compounds—ethyl alcohol and isopropyl alcohol—that have generally underrated germicidal characteristics 482. FDA has not cleared any liquid chemical sterilant or high-level disinfectant with alcohol as the main active ingredient. These alcohols are rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria; they also are tuberculocidal, fungicidal, and virucidal but do not destroy bacterial spores. Their cidal activity drops sharply when diluted below 50% concentration, and the optimum bactericidal concentration is 60%-90% solutions in water (volume/volume) 483, 484.
Mode of Action
The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. This mechanism is supported by the observation that absolute ethyl alcohol, a dehydrating agent, is less bactericidal than mixtures of alcohol and water because proteins are denatured more quickly in the presence of water 484, 485. Protein denaturation also is consistent with observations that alcohol destroys the dehydrogenases of Escherichia coli 486, and that ethyl alcohol increases the lag phase of Enterobacter aerogenes 487 and that the lag phase effect could be reversed by adding certain amino acids. The bacteriostatic action was believed caused by inhibition of the production of metabolites essential for rapid cell division.
Bạn đang xem: Chemical Disinfectants
Microbicidal Activity
Methyl alcohol (methanol) has the weakest bactericidal action of the alcohols and thus seldom is used in healthcare 488. The bactericidal activity of various concentrations of ethyl alcohol (ethanol) was examined against a variety of microorganisms in exposure periods ranging from 10 seconds to 1 hour 483. Pseudomonas aeruginosa was killed in 10 seconds by all concentrations of ethanol from 30% to 100% (v/v), and Serratia marcescens, E, coli and Salmonella typhosa were killed in 10 seconds by all concentrations of ethanol from 40% to 100%. The gram-positive organisms Staphylococcus aureus and Streptococcus pyogenes were slightly more resistant, being killed in 10 seconds by ethyl alcohol concentrations of 60%-95%. Isopropyl alcohol (isopropanol) was slightly more bactericidal than ethyl alcohol for E. coli and S. aureus 489.
Xem thêm : Urinary Tract Infections and the Role of Nonprescription Products
Ethyl alcohol, at concentrations of 60%-80%, is a potent virucidal agent inactivating all of the lipophilic viruses (e.g., herpes, vaccinia, and influenza virus) and many hydrophilic viruses (e.g., adenovirus, enterovirus, rhinovirus, and rotaviruses but not hepatitis A virus (HAV) 58 or poliovirus) 49. Isopropyl alcohol is not active against the nonlipid enteroviruses but is fully active against the lipid viruses 72. Studies also have demonstrated the ability of ethyl and isopropyl alcohol to inactivate the hepatitis B virus(HBV) 224, 225 and the herpes virus, 490 and ethyl alcohol to inactivate human immunodeficiency virus (HIV) 227, rotavirus, echovirus, and astrovirus 491.
In tests of the effect of ethyl alcohol against M. tuberculosis, 95% ethanol killed the tubercle bacilli in sputum or water suspension within 15 seconds 492. In 1964, Spaulding stated that alcohols were the germicide of choice for tuberculocidal activity, and they should be the standard by which all other tuberculocides are compared. For example, he compared the tuberculocidal activity of iodophor (450 ppm), a substituted phenol (3%), and isopropanol (70%/volume) using the mucin-loop test (106 M. tuberculosis per loop) and determined the contact times needed for complete destruction were 120-180 minutes, 45-60 minutes, and 5 minutes, respectively. The mucin-loop test is a severe test developed to produce long survival times. Thus, these figures should not be extrapolated to the exposure times needed when these germicides are used on medical or surgical material 482.
Ethyl alcohol (70%) was the most effective concentration for killing the tissue phase of Cryptococcus neoformans, Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum and the culture phases of the latter three organisms aerosolized onto various surfaces. The culture phase was more resistant to the action of ethyl alcohol and required about 20 minutes to disinfect the contaminated surface, compared with <1 minute for the tissue phase 493, 494.
Xem thêm : Garden of Life
Isopropyl alcohol (20%) is effective in killing the cysts of Acanthamoeba culbertsoni (560) as are chlorhexidine, hydrogen peroxide, and thimerosal 496.
Uses
Alcohols are not recommended for sterilizing medical and surgical materials principally because they lack sporicidal action and they cannot penetrate protein-rich materials. Fatal postoperative wound infections with Clostridium have occurred when alcohols were used to sterilize surgical instruments contaminated with bacterial spores 497. Alcohols have been used effectively to disinfect oral and rectal thermometers498, 499, hospital pagers 500, scissors 501, and stethoscopes 502. Alcohols have been used to disinfect fiberoptic endoscopes 503, 504 but failure of this disinfectant have lead to infection 280, 505. Alcohol towelettes have been used for years to disinfect small surfaces such as rubber stoppers of multiple-dose medication vials or vaccine bottles. Furthermore, alcohol occasionally is used to disinfect external surfaces of equipment (e.g., stethoscopes, ventilators, manual ventilation bags) 506, CPR manikins 507, ultrasound instruments 508 or medication preparation areas. Two studies demonstrated the effectiveness of 70% isopropyl alcohol to disinfect reusable transducer heads in a controlled environment 509, 510. In contrast, three bloodstream infection outbreaks have been described when alcohol was used to disinfect transducer heads in an intensive-care setting 511.
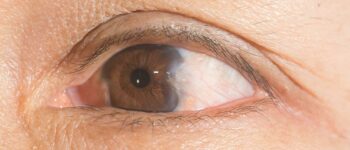
The documented shortcomings of alcohols on equipment are that they damage the shellac mountings of lensed instruments, tend to swell and harden rubber and certain plastic tubing after prolonged and repeated use, bleach rubber and plastic tiles 482 and damage tonometer tips (by deterioration of the glue) after the equivalent of 1 working year of routine use 512. Tonometer biprisms soaked in alcohol for 4 days developed rough front surfaces that potentially could cause corneal damage; this appeared to be caused by weakening of the cementing substances used to fabricate the biprisms 513. Corneal opacification has been reported when tonometer tips were swabbed with alcohol immediately before measurement of intraocular pressure 514. Alcohols are flammable and consequently must be stored in a cool, well-ventilated area. They also evaporate rapidly, making extended exposure time difficult to achieve unless the items are immersed.
Nguồn: https://buycookiesonline.eu
Danh mục: Info