Prim Care Companion CNS Disord 2021;23(6):20f02871
To cite: Petriceks AH, Stern TA. Assessment of neurologic signs and symptoms: establishing a diagnosis of multiple sclerosis. Prim Care Companion CNS Disord. 2021;23(6):20f02871. To share: https://doi.org/10.4088/PCC.20f02871
Bạn đang xem: Assessment of Neurologic Signs and Symptoms: Establishing a Diagnosis of Multiple Sclerosis
© Copyright 2021 Physicians Postgraduate Press, Inc.
aHarvard Medical School, Boston, Massachusetts bDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts *Corresponding author: Aldis H. Petriceks, BA, Harvard Medical School, 25 Shattuck St, Boston, MA 02115 ([email protected]).
Have you been uncertain about how you can make a diagnosis of multiple sclerosis by reviewing signs and symptoms, results of laboratory tests, and brain imaging? Have you wondered which treatments are available for acute or chronic symptoms of multiple sclerosis? Have you puzzled over which (and how) psychiatric and cognitive symptoms of multiple sclerosis can best be treated? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms A, a 34-year-old woman with no known medical conditions, presented with 2 days of vision loss and pain with movement of her left eye, as well as fatigue and muscle weakness. At first, she attributed her symptoms to decreased caloric intake associated with a new diet. Then, she attributed her eye pain to working long hours on a computer. However, after developing urinary symptoms and suffering near falls at work, she had a coworker bring her to the emergency department (ED). In the ED, her motor examination revealed 3/5 strength in her right arm and leg, with 5/5 strength on the left, while there was decreased sensation to vibration in the right arm and leg. Visual acuity was decreased in the left eye and was normal in the right eye. Babinski sign was positive in the right foot. A neurologist was consulted.
DISCUSSION
What Is Multiple Sclerosis?
Multiple sclerosis (MS) is an immune-mediated degenerative disease of the central nervous system (CNS) in which host immune cells contribute to demyelination of axons in the gray and white matter.1 This process of immune-mediated inflammation and damage is associated with a variety of radiographic findings and neurologic symptoms.2 None of these symptoms are totally specific to MS, but their combination and correlation with neuroimaging findings allow for making a diagnosis of MS.3
MS is driven by inflammatory processes in the CNS that involve autoreactive lymphocytes (T- and B-cells), depletion of oligodendrocytes (that normally produce the myelin sheath that allow for saltatory conduction throughout axons), and, ultimately, the degradation of axons in the CNS. These inflammatory, immunologic, and degenerative processes are tightly interconnected.
Four hypotheses have been generated to help explain this relationship in MS.4 The first states that autoreactive lymphocytes cause inflammation in the CNS, which directly precipitate demyelination and axonal degeneration. The second hypothesis states that some neurodegenerative process occurs prior to the inflammation, exposing the CNS to further degeneration as autoreactive lymphocytes attack vulnerable axons. The third hypothesis states that these inflammatory and neurodegenerative processes contribute equally, or simultaneously, to the pathophysiologic picture of MS, but occur independently of one another. The fourth hypothesis states that autoreactive lymphocytes in the CNS cause inflammation, but this inflammation serves primarily to expose an “intrinsic neurodegenerative susceptibility,” which then, independently of the lymphocytic activity, leads to further damage.4 The loss of axons and neurons is central to MS, which often begins with focal inflammatory lesions. These lesions can occur anywhere in the CNS (eg, in the cerebral cortex, the optic nerve, the spinal cord) but are found primarily in the white matter.
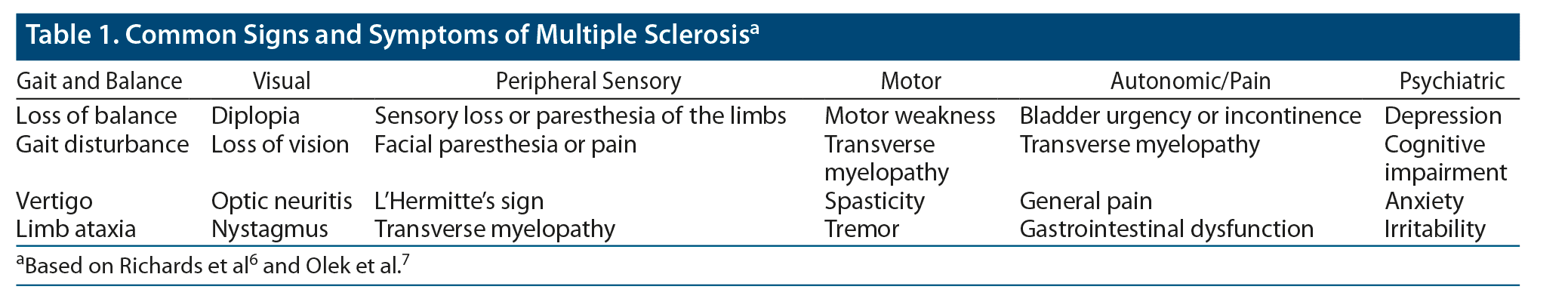
MS is defined by clinical attacks that are disseminated in time and space, meaning that episodes arise at different times and can be localized to different regions of the CNS.5 The signs and symptoms experienced by any one patient (or in any one episode) are determined by the location and extent of a lesion and by the number of lesions. The most prominent manifestations are sensory loss or paresthesia in the limbs; diplopia, loss of vision, or visual disturbance; acute or subacute motor weakness; gait disturbance; loss of balance; facial paresthesia or pain; L’Hermitte’s sign (the experience of shock-like sensations through the back, limbs, or both, secondary to neck flexion); vertigo; bladder urgency or incontinence; limb ataxia; transverse myelopathy; general pain; and psychiatric symptoms, most notably depression (Table 1).6,7
While the full list of possible manifestations of MS would not be expected in any given episode or even over the course of a lifetime for any given patient, none of these symptoms, in isolation, unambiguously indicates MS. Rather, it is the collection of symptoms that co-occur—and the demonstration of multiple episodes of symptom onset and remittance—that suggests MS.
In addition to clinical symptoms and signs, MS is distinguished by the finding of focal hyperintensities on T2- or proton-weighted magnetic resonance imaging (MRI).8 These lesions are often round or oval and are as large as 1-2 cm in diameter.8 They can be found throughout the CNS but are most often located in the white matter. Periventricular lesions are suggestive of MS, especially those aligned perpendicular to the corpus collosum (known as “Dawson’s fingers”).8 Juxtacortical, infratentorial, and spinal cord lesions are also common in MS.9
What Is the Natural Course of MS?
There are 2 main “phenotypes” of MS: relapsing-remitting and progressive. Relapsing-remitting MS (RRMS) begins with a clinically isolated syndrome (CIS), in which one or several manifestations of MS arise for the first time in a patient not diagnosed with MS.3,10 More than 80% of patients with MS present with CIS, and many have signs and symptoms of acute unilateral optic neuritis (eg, loss of vision, periorbital pain, color deficits, visual field deficits).4,11,12 CIS often develops acutely or subacutely and persists for at least 24 hours; it is not associated with fever or infection.10
If a patient suffers a second attack—a “relapse”—after resolution of the CIS, they meet criteria for RRMS.4 Patients who present with CIS and show evidence of white matter lesions at sites not implicated by their CIS symptoms face a 50% likelihood of relapse within 2 years, and 82% risk relapse within 20 years. Relapses typically occur on an irregular basis (but often arise more than once per year), and symptoms worsen over hours to days.4,13
Patients with RRMS typically suffer cumulative neurologic and psychiatric symptoms as a result of continued relapses.4 Many develop some degree of residual disability between active flares.14 A significant percentage—approximately 65%—shift from the RRMS pattern to a steady progression of neurologic manifestations and disability (with or without discrete “relapses” of more intense manifestations).4 This is known as secondary-progressive MS (SPMS).
The progression from RRMS to SPMS typically occurs over the course of decades.4 In 1 long-term follow-up study, patients who eventually developed SPMS transitioned to the progressive disease within 19 years (median) following the CIS and 12 years following their diagnosis of MS.15 However, there are no absolute criteria for determining the end of RRMS and the onset of SPMS. The latter is a retrospective diagnosis; the transition is a gradual one devoid of known triggers, events, or clinicopathologic correlates.14
Xem thêm : The Blog
Another form of progressive MS exists that does not begin with CIS; it is known as primary-progressive MS (PPMS), a subtype that encompasses 10%-15% of patients with MS.13 Patients with PPMS experience a steady, gradual onset of signs and symptoms that progress over months to years.13 Approximately 80% of these patients present with progressive, spastic, partial paralysis of the limbs, most often the lower limbs.13 Patients may have difficulty walking due to weakness or problems with balance. Sensory, bladder, and sphincter dysfunction are also common, as is exertional fatigue.13
Overall, progression of MS is characterized by the presence and frequency of clinical relapses (in RRMS), functional disability, and evidence of new or enlarging lesions on T2-weighted MRI. The pattern of neurologic and functional impairment is determined by each patient’s unique set of accumulated CNS lesions and clinical deficits. Patients with an increased number of lesions at baseline, and those with greater total lesion volume within 5 years of the CIS, tend to experience more disability 20 years after disease onset.3 Those who receive disease-modifying treatment between the onset of CIS and the first relapse may have longer remittance before the initial relapse, as well as lower overall disability.16,17
Unfortunately, MS remains an incurable disease, with challenging implications for lifespan and quality of life. One Danish study found that individuals diagnosed with MS face a 10-year reduction in life expectancy when compared to those in the general (age-matched) population.18 Death in patients with MS is often attributed to complications of the disease, such as increased susceptibility to bladder infections in the elderly.4 Patients with MS also experience high rates of depression and an increased risk for suicide attempts.4 Clinicians should also be aware of the tremendous psychosocial burden having MS places on patients and families.
What Causes MS?
The exact etiology of MS has not been established. While genetic susceptibility and the environment interact to place certain individuals at increased risk,4 MS tends to be more prevalent farther from the equator, perhaps due in part to differential patterns of exposure to sunlight.2,4 Individuals who migrate from high-risk to low-risk regions during childhood tend to have a lower risk of MS compared to their original population, while the opposite holds for those who migrate from low-risk to high-risk regions.4 Other environmental factors include cigarette smoking, obesity, and later exposure to certain infectious pathogens.3,4
Genetics also appear to play a sizeable role in the development of MS. Females account for approximately 75% of cases.2 Those who have a first-degree relative with MS face up to a 4% risk, compared to approximately 0.1% of those in the general population.2 Many of the genes implicated in MS code for proteins involved in immune cells; however, there do not appear to be reliable genetic markers for predicting the clinical course or response to treatment for any individual with MS.2
What Is the Differential Diagnosis for MS?
The wide range of signs and symptoms in MS makes the establishment of a concise and clear differential diagnosis difficult. More helpful may be the delineation of broad types of diseases, alongside specific approaches that one might use to rule out or rule in these disease categories in cases of suspected MS.
A wide range of inflammatory conditions (eg, acute disseminated encephalomyelitis), demyelinating diseases (eg, Guillain-Barre syndrome), systemic and CNS infections (eg, bacterial meningitis, progressive multifocal leukoencephalopathy), granulomatous diseases (eg, sarcoidosis), and other conditions (eg, B12 deficiency, compressive spinal cord lesions) should be on the broad differential when a patient presents with suspected CIS.19 More comprehensive differential diagnoses may be found in the literature.19,20
During the assessment, it is important to consider “red flag” signs and symptoms suggestive of diseases other than MS.21 The list of these signs, symptoms, and presentations is wide and covered in detail in the literature.19 For most clinicians, however, the differential is narrowed when a patient has had 2 or more distinct episodes of symptoms suspicious for MS, with periods of recovery in between.
How Can MS Be Evaluated?
Clinicians evaluating possible MS should begin with a detailed history and physical examination. The history should focus on the nature of the presenting symptoms, as well as on previous clinical attacks. Clinicians should ask about prior infections, as well as the onset and duration of symptoms. Past medical and family history should include any neurologic or autoimmune conditions.
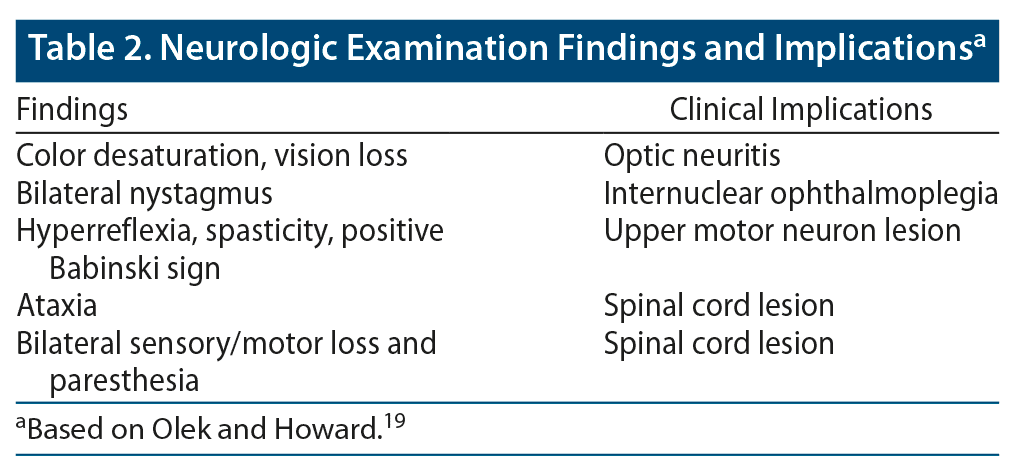
A thorough neurologic examination should be conducted. Depending on the nature of the presenting concerns (and whether the patient is actively experiencing symptoms), possible findings may include color desaturation and vision loss (suggestive of optic neuritis), bilateral nystagmus (suggestive of internuclear ophthalmoplegia), signs of upper motor lesions (eg, hyperreflexia, spasticity, positive Babinski sign), ataxia (suggestive of cerebellar involvement), wide gait or other gait disturbances (also suggestive of cerebellar involvement), hemisensory loss (suggestive of unilateral cortical lesion), and bilateral sensory loss and paresthesia (suggestive of a spinal cord lesion) (Table 2).19
Any patient being evaluated for MS should undergo a brain MRI with and without contrast.22 If MRI findings are inconclusive, the patient may also receive additional testing to support a diagnosis of MS.
Cerebrospinal fluid (CSF) analysis is the primary ancillary test. Analysis for oligoclonal immunoglobulin G (IgG) is of primary importance. In a meta-analysis,23 87.7% of 12,253 patients with MS had oligoclonal bands (OCB) in the CSF. However, given the invasiveness of a lumbar puncture and the reliability of clinical and MRI findings, a lumbar puncture is not necessary in the initial workup.
Taking clinical, imaging, and laboratory data into account, clinicians should refer to the revised 2017 McDonald criteria when making a final diagnosis.10 The McDonald criteria—based on the number of attacks described in the history, as well as the number of objective MS lesions found on neuroimaging—are most often applied to patients who present with findings suggestive of a CIS or relapse (Figure 1). A patient must have evidence of dissemination in space and time to meet the McDonald criteria for a diagnosis of MS, meaning that there must be evidence of multiple attacks (or the diagnostic equivalent, addressed here) and multiple MS lesions. The following scenarios illustrate the potential supportive evidence for meeting these criteria.
First, a patient could present with 1 clinical attack with objective findings suspicious for MS and demonstrate evidence of 1 MS lesion on MRI. Such a patient would not meet criteria for MS, having dissemination in neither space nor time. However, a diagnosis could be made using additional data. Dissemination in space could be shown by a later, additional clinical attack implicating a site in the CNS not implicated in the first attack or by later or concurrent MRI evidence of an additional MS lesion.3 Dissemination in time could be demonstrated by an additional clinical attack (regardless of the site implicated), later MRI evidence of an additional MS lesion, or the presence of OCB on CSF analysis. Any combination of additional findings that fulfill criteria for both dissemination in space and dissemination in time would qualify for a diagnosis of MS.
Second, a patient could present with 1 clinical attack with objective findings suggestive of MS and demonstrate evidence of 2 or more MS lesions on an MRI scan. The MRI findings would meet criteria for dissemination in space, while the clinical findings would not meet criteria for dissemination in time. As in the first case, dissemination in time could be demonstrated by later evidence of an additional clinical attack, or by later (but not concurrent) evidence of an additional MS lesion on an MRI scan, or by the presence of OCB on CSF analysis.3 If any of these 3 findings was discovered, criteria would be met for dissemination in space and time.
Third, a patient could present with 2 or more clinical attacks with objective findings suggestive of MS and demonstrate evidence of 1 MS lesion on an MRI scan. In this case, the clinical history would fulfill criteria for dissemination in time. Dissemination in space could then be demonstrated by an additional, later attack that implicated a CNS site not implicated in previous attacks or by an additional lesion found on an MRI scan.3 Alternatively, criteria for dissemination in space could also be fulfilled by reasonable historical evidence of a previous attack implicating an additional CNS site.
Xem thêm : Toner Vs. Witch Hazel Vs. Rubbing Alcohol
Finally, a patient could present with 2 or more clinical attacks with objective findings suggestive of MS and demonstrate evidence of 2 more MS lesions on a MRI scan. This patient would meet criteria for both dissemination in space and dissemination in time and require no further evidence for a diagnosis of MS.
How Is MS Managed in Acute and Chronic Settings?
The pharmacologic treatment of MS can be categorized into 2 broad components: treatment of acute relapsing symptoms and long-term, disease-modifying therapy. Treatment of acute MS relapse targets timely resolution of symptoms. High-dose methylprednisolone is often the therapy of choice for this scenario.24 Plasma exchange therapy may also be used for steroid-resistant clinical attacks.25 Disease-modifying therapy targets the adaptive immune system to reduce neuroinflammation.3
Broadly speaking, the disease-modifying medications are applied in 1 of 2 frameworks. The first is labeled “escalation strategy.” It consists of initial treatment with a moderately effective, safer, less expensive drug, followed by later treatment with potentially more effective but more expensive and potentially less safe options.3 This approach may be appropriate for patients early in their disease progression or with stable rates of relapse. In those with debilitating or unpredictable disease, the “induction strategy” may be more suitable. This strategy involves initial therapy with a highly effective treatment, followed by either a long-term remission (in the ideal scenario) or step-down to a less effective, but safer, treatment option.3
It is important that clinicians understand the utility of early and effective treatment of MS. According to a review, patients who receive disease-modifying therapy between their first and second clinical attacks have a lower risk of accumulating moderate levels of disability.3 In general, there are consistent and numerous clinical benefits to initiation of disease-modifying treatment early in the disease course.3 However, treatment of acute relapses does not appear to have long-term prognostic impact on MS, and the goal in this scenario is to promote acute recovery and symptom relief.3
In 2018, the American Academy of Neurology released practice guideline recommendations for disease-modifying therapies in adults with MS.26 Clinicians caring for patients with MS may refer to these recommendations for detailed insights on specific treatment regimens.
How Does MS Affect Pregnancy, and How Does Being Pregnant Impact a Patient’s Experience of MS?
Because MS is an immune-mediated disease that mostly affects genetic and anatomic females—many of whom develop the disease during reproductive age—clinicians should be aware of the relationship between MS and pregnancy.2 In general, having MS does not appear to negatively impact fertility or the ability to become pregnant and deliver a healthy child.5 Also, the rate of relapse tends to decline during each trimester of the pregnancy.4 Breastfeeding and epidural anesthesia do not appear to impact the disease, though breastfeeding in particular is still an area of research interest in the course of MS.4,27 It should be noted, however, that rates of relapse may increase by a factor of 3 in the weeks immediately following delivery.4
What Are the Psychological and Psychiatric Consequences of MS and Its Treatment?
Patients with MS face a bevy of psychological and psychiatric challenges. In 1 study28 of patients with MS, suicide represented a 7.5-fold greater rate of deaths than in the age-matched controls. A separate review29 found that 15.7% of patients with MS experience depression in a given 12-month period, nearly double that of the general population. The lifetime risk for major depressive disorder (MDD) may be as high as 54% in these patients, and the prevalence of depressive symptoms may be much higher.29 The cause of this high prevalence is likely multifactorial, influenced by the neurologic consequences of MS, the psychosocial stressors of acute relapse and accumulated disability, and the impact of treatment.29,30
Management of MDD and depressive symptoms in those with MS is not fundamentally different than treatment in patients without MS. The former group (in addition to the latter) is underrecognized for its psychiatric suffering, despite the fact that this suffering is amenable to psychotherapy and pharmacologic treatment.29,31 The predominant pharmacologic options include use of selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs).30
Cognitive-behavioral therapy (CBT) is the primary psychotherapeutic method for treating depression in patients with MS. Studies29,32 suggest that response rates to CBT approach or exceed those of pharmacologic interventions. Furthermore, rates of attrition are much lower.29
Other psychiatric conditions faced by patients with MS include anxiety disorders, substance use disorders, and pseudobulbar effects.29 Combinations of psychotherapy and pharmacotherapy are also helpful.
Are There Specific Measures by Which Clinicians Can Assess Response to Treatment?
As treatments for MS have progressed, many clinicians, researchers, and patients have moved their goals of care beyond the reduction of relapse rates and control of disease sequelae to aim for “no evident disease activity” (NEDA). NEDA is a concept that involves 3 primary markers of minimal MS disease activity.33 First, patients with NEDA should have no evidence of relapses. Second, patients should demonstrate no progression of accumulated disability secondary to MS. Third, patients should not demonstrate new or progressing CNS lesions on MRI.
These markers of treatment success in MS represent a promising approach for clinicians. However, it remains to be seen how NEDA should be implemented in clinical practice. For instance, Stangel et al34 note that these broad markers do not allow for slight changes in disability progression, clinical signs and symptoms, or other neuropsychological changes, which could theoretically occur in patients without active relapse and at the very least represent an acceptable and “successful” treatment outcome for many patients. The authors34 argue that factors such as the ability to work and quality of life, among others, should be included in the assessment. They propose 4 domains to assess for in the determination of NEDA. The domains are composed of the original 3 NEDA components, alongside a domain for “neuropsychological parameter and patient-related outcomes.” Specific components of the assessments for each of these 4 domains can be found in the original article.34 If a particular domain is found, by the standards noted by the authors,34 to have sufficiently low level of activity or progression, it is considered “green.” Stangel et al34 suggest a treatment period of 6 months, after which success or failure of treatment should be assessed. If each of the 4 domains is determined to be green, treatment is considered successful, no change is indicated, and the patient should be reevaluated in 6 months. If 1 domain is “yellow,” indicating a slight change in condition, reevaluation should take place in 3 months or less. If 2 or more domains are yellow, or at least 1 domain is “red” (meaning severe change), the clinician should consider treatment modification.
What Are the Roles of Physical Therapy, Occupational Therapy, and Lifestyle Modifications in Patients With MS?
Exercise has proved effective in promoting greater mobility, positive mood, and quality of life in patients with MS.35,36 Combined with physical therapy, it may contribute to partial relief of symptoms, including muscle spasticity, fatigue, ambulatory difficulties, ataxia, tremor, and pain.3 Patients with MS can be cared for in a multidisciplinary holistic fashion, incorporating support from physical therapists, occupational therapists, and mental health professionals.
What Happened to Ms A?
The neurologist in the ED suspected that Ms A was suffering a CIS and was at risk for a future relapse of MS. A lumbar puncture was performed; the CSF analysis was positive for OCB. A brain MRI showed several ovoid white matter lesions indicative of MS. Checking the McDonald criteria, the neurologist found that Ms A showed evidence of dissemination in space (with her multiple lesions on MRI) and that dissemination in time was fulfilled by the combination of 1 clinical attack plus a CSF analysis positive for OCB. Ms A was diagnosed with MS and started on a trial of intravenous (IV) methylprednisolone. Her symptoms resolved over the next few days, and she was referred to a neurologist for ongoing outpatient management.
In the neurologist’s office, Ms A was concerned about the long-term effects of her MS, endorsing anxiety and depression about her professional and personal future. She worried about embarrassing episodes of urinary incontinence like the one she experienced with her CIS. As a successful employee in a large accounting firm, she feared that any accumulated neurologic disability would limit her accomplishments. She expressed a bleak outlook on life and felt that her diagnosis had “ruined everything.”
The neurologist responded by describing a thorough, patient-centered management approach that targeted her medical and psychosocial concerns. She told Ms A that early initiation of disease-modifying therapy can benefit patients with MS over the long term. They agreed on an escalation strategy and that Ms A would start seeing a psychiatrist for an assessment of her symptoms of anxiety and depression. Ms A was open to CBT and said she would consider medications if CBT alone was not effective. Finally, Ms A was counseled on physical therapy and occupational therapy options. She decided to be proactive about management of her MS and hired a personal trainer. After the initial visit to the neurologist, Ms A was still wary about her future, but confident in her ability to live a meaningful life.
Submitted: November 17, 2020; accepted February 18, 2021. Published online: December 23, 2021. Potential conflicts of interest: Dr Stern has received royalties from Elsevier and the Massachusetts General Hospital Psychiatry Academy. Mr Petriceks reports no conflicts of interest related to the subject of this article. Funding/support: None.
Clinical Points
- Multiple sclerosis (MS) is an incurable autoimmune condition of the central nervous system associated with neurologic disability, a variety of radiographic findings, psychiatric suffering, and a shortened life expectancy.
- There are 2 main phenotypes of MS: relapsing-remitting and progressive.
- The pharmacologic treatment of MS involves treatment of acute relapsing symptoms and long-term, disease-modifying therapy.
- Patients with MS face many psychological and psychiatric challenges.
Nguồn: https://buycookiesonline.eu
Danh mục: Info