Background There is debate about combining benzodiazepines with selective serotonin reuptake inhibitors in the acute treatment of panic disorder. Although this medication combination is widely used in clinical practice, there is no well-tested, optimal method of coadministering these medications for the treatment of panic disorder. The purpose of this study was to test the efficacy of early coadministration of clonazepam with sertraline in the treatment of panic disorder.
Methods Fifty patients with panic disorder were randomized into a double-blind clinical trial. Patients received open-label sertraline for 12 weeks (target dose, 100 mg/d), and in addition were randomized to groups receiving either 0.5 mg of active clonazepam 3 times daily or placebo clonazepam for the first 4 weeks of the trial. The clonazepam dose was then tapered during 3 weeks and discontinued.
Bạn đang xem: Early Coadministration of Clonazepam With Sertraline for Panic Disorder
Results Thirty-four (68%) of 50 patients completed the trial. Drop-out rates were similar in the sertraline/placebo vs the sertraline/clonazepam group (38% vs 25%) (P = .5). An intent-to-treat analysis (on last observation carried forward data) revealed a much greater proportion of responders in the sertraline/clonazepam compared with the sertraline/placebo group at the end of week 1 of the trial (41% vs 4%) (P = .003). There was also a significant between-group difference at the end of week 3 with 14 (63%) of 22 of the sertraline/clonazepam group responding to treatment vs 8 (32%) of 25 of the sertraline/placebo group (P = .05). This difference was not observed at other times during the trial.
Conclusion These data indicate that rapid stabilization of panic symptoms can be safely achieved with a sertraline/clonazepam combination, supporting the clinical utility of this type of regimen for facilitating early improvement of panic symptoms relative to sertraline alone.
COMBINING benzodiazepines with selective serotonin reuptake inhibitors (SSRIs) and other antidepressant-like agents during the course of treatment for panic disorder seems to be a common clinical practice.1 However, there are few controlled studies to guide the physician on how to best accomplish this. A previous study by our group documented the early antipanic efficacy of an imipramine/alprazolam regimen for the stabilization treatment of panic disorder.2 However, in this trial, the alprazolam dosage proved difficult to taper, which in turn had a negative ongoing effect on clinical state in the combination treatment group. Other researchers, using a naturalistic study design, have begun to report evidence of early antipanic efficacy of a paroxetine/clonazepam coadministration strategy,3 with uneventful tapering of this benzodiazepine regimen. Interim results of a controlled study by another group, examining the additive effects of either brief or sustained combined treatment with clonazepam in patients with panic disorder receiving paroxetine, suggested that the benefits of combined treatment are primarily immediate (e-mail communication, Mark H. Pollack, MD, Harvard University, Cambridge, Mass, 2001). However, more firm conclusions await completion of this trial. While there are few controlled data on the optimal use of benzodiazepines in conjunction with SSRIs for panic, this treatment strategy shows promise as a reliable and safe approach for the stabilization of acute panic disorder.
Accordingly, the purpose of our study was to determine, in a controlled manner, whether early coadministration of the benzodiazepine, clonazepam, with the SSRI, sertraline, would facilitate rapid clinical stabilization of panic symptoms relative to the administration of sertraline alone. We hypothesized that with the combination strategy, early improvements in the core symptoms of panic disorder such as panic attacks, distress related to panic attacks, anticipatory anxiety, and agoraphobia would be superior without excessive short- or long-term adverse events or loss of early treatment gains.
The study was a double-blind placebo-controlled trial of the efficacy of early coadministration of clonazepam with sertraline for the treatment of active panic disorder. Panic disorder was diagnosed according to the DSM-IV criteria.4 Patients (N = 50) were treated with flexible-dose, open-label sertraline (target dose, 100 mg/d) for 12 weeks. In addition, for the first 4 weeks of the trial, patients were randomly assigned to receive either placebo clonazepam (n = 26) or active clonazepam (0.5 mg by mouth 3 times daily) (n = 24), followed by 3 weeks of tapered doses. After the tapering period, open-label sertraline treatment was continued through the end of the study. We adopted a design that would, as much as possible, model clinical practice. Patients received support as a part of their pharmacotherapy sessions (advice about medications and their adverse effects, information about panic disorder) during the 12-week study period, but did not receive cognitive behavioral therapy or any other psychotherapy at this time.
The study was conducted at the offices of the Yale Anxiety Clinic in New Haven and Hartford, Conn. After a psychiatric evaluation performed by a research psychiatrist (A.W.G., A.A., P.J.), patients were informed of the study rationale and procedures. All patients gave their written consent to participate in the study and received a copy of the approved informed consent document for their records.
Xem thêm : Hemorroides
To be eligible for the study, patients had to be aged 18 years or older and were required to have a weekly panic attack frequency of 1 or more per week during the month prior to intake into the clinic, and at least 1 full panic attack during the week prior to randomization. Each patient was given a physical examination and routine laboratory tests were performed. Urine toxicology results at intake were negative in each case for substances of abuse (mean ± SD time that urine was tested prior to randomization, 11 ± 15 days). The work-up also included urinalysis, electrocardiography, liver and thyroid function tests, blood cell count, and levels of serum electrolytes, glucose, and gonadotropin (for women). The current, principal diagnosis of panic disorder with or without agoraphobia was confirmed by administration of a semistructured diagnostic interview based on the DSM-IV (Structured Clinical Interview for DSM-IV, Anxiety Disorder Interview Schedule, or Mini International Neuropsychiatric Interview Plus).5-7 Patients with mild current major depression were eligible for the study provided that the depression was not more clinically prominent than the panic disorder. Patients were required to not have taken psychotropic medications for at least 2 weeks prior to randomization. No patients had received long half-life benzodiazepines such as flurazepam, diazepam, or chlordiazepoxide prior to the study, and 1 had taken 0.5 mg of clonazepam as needed until 4 weeks prior to the study. One patient had been taking fluoxetine until approximately 12 months prior to intake. Subjects were excluded if they had a lifetime history of a psychotic disorder, a bipolar disorder, or a condition affecting central nervous system function (eg, epilepsy, severe head injury, meningitis). Women who were pregnant or lactating were excluded, as were women of childbearing potential who were not using an acceptable method of contraception (oral contraceptive, intrauterine device, progesterone implants or injections, barrier methods, and cream). In addition, patients were excluded if they had a substance abuse disorder within 6 months of the diagnostic interview, had previously failed an adequate trial of sertraline or clonazepam, or were allergic to either drug in the past. Patients with a suicide plan or suicidal behavior during the month prior to intake were not eligible and were referred for ongoing clinical care.
There were 13 study visits (1 baseline and 12 subsequent weekly visits) at which efficacy and safety measures were acquired. The primary efficacy measures were (1) mean scores of clinician-assessed weekly panic attack frequency (item 1 [0-4 rating] of the 7-item Panic Disorder Severity Scale [PDSS]8); (2) percentage of patients responding to treatment by visit (responders had to have a decrease of 50% or more from their baseline PDSS sum score); (3) mean change from baseline scores of patient-rated weekly panic attack frequency; and (4) mean PDSS sum score (scoring range, 0-28). The PDSS samples several symptom domains of panic disorder, including frequency of panic attacks (limited and full panic attacks), distress during panic attacks, agoraphobic symptoms, anticipatory anxiety, and social and occupational functioning. The PDSS has become a standard panic severity assessment tool.9-11 Secondary efficacy variables included mean scores of the Hamilton Anxiety Scale (HAM-A),12 Clinical Global Impression of Illness Severity (CGI-S),13 and the Sheehan Clinician-Rated Anxiety Scale (CRAS).14 A CGI Improvement scale (CGI-I)13 was also administered weekly, beginning the week after randomization. Safety was monitored clinically at each visit by a study psychiatrist. At each visit, patients filled out an adverse symptom checklist (29 items, each item rated 0-3 [0 = absent; 1 = slight; 2 = moderate; 3 = severe])15 and handed in a weekly panic attack diary.2 Patients recorded the duration, intensity, number, and type of symptoms of each panic attack they experienced. They were instructed on the use of the diary instrument by research staff and were asked to log their panic attacks at the end of each day and return the completed diary at each study visit. The prescribing psychiatrist reviewed the diary at each visit to check that patients were monitoring correctly, and to assist in the evaluation of the patient’s clinical status. Another urine drug screen was conducted at the end of week 12 of treatment in 21 of 34 patients who completed the study. None of the follow-up urine test results indicated surreptitious use of benzodiazepines or any other substances of abuse.
At the first treatment visit (randomization visit) patients started receiving open-label sertraline, one 25-mg tablet by mouth daily. The dose was titrated to 50 mg/d at the second visit (end of week 1), and finally to 100 mg/d by the third visit (end of week 2 of treatment). The 100-mg daily dose level was the target dose of sertraline, selected on the basis of previous literature indicating that most patients with panic disorder will respond to 50 to 100 mg/d of sertraline.10,16,17 The dose of sertraline was kept constant after the end of the third week of the study. Patients were required to receive a minimum daily dose of 50 mg to remain in the study. In addition, at visit 1, patients were randomly assigned (from a computer-generated randomization schedule) to either active clonazepam, 0.5-mg capsules by mouth 3 times daily (1.5 mg/d), or 1 placebo capsule by mouth 3 times daily. The capsules were identical in appearance. If the clonazepam/placebo caused excessive sedation, the dose was reduced to 1 capsule 2 times daily. After 4 weeks of treatment with the sertraline/clonazepam combination (at visit 5, end of week 4 of the trial), we tapered the doses for patients receiving the clonazepam/placebo over a 3-week period. During the first week of the taper patients took one 0.5-mg capsule 2 times daily, followed by one 0.5-mg capsule daily during the second week. The dose was then decreased to one 0.25-mg capsule daily for the third week before discontinuation. Open-label sertraline treatment was maintained for an additional 5 weeks following the taper of clonazepam.
We compared the 2 intent-to-treat (ITT) study groups for baseline differences on key demographic (age, sex, race), clinical (percentage of patients with agoraphobia, percentage of patients with major depression, mean duration of illness in years), and efficacy measures (PDSS sum scores, PDSS weekly panic frequency-item 1, patient’s diary of weekly panic frequency, HAM-A, CRAS, CGI Severity). For the baseline analyses, independent t tests were used to compare the groups on continuous measures and Fisher exact test on categorical measures. For the primary categorical efficacy measure of response status (defined as a 50% decrease from baseline PDSS sum scores), we conducted Fisher exact analyses, comparing the treatment groups at each time point. All other continuous efficacy measures were subjected to an omnibus multivariate analysis of variance (MANOVA) (general linear model),18 using the Pillais trace statistic.19 The MANOVA was considered a conservative first step in establishing the presence of an effect in the whole data set, since it protects against type I error inflation by simultaneously analyzing all measures taken at all time points on all subjects.20 In addition, it has the advantage of making fewer assumptions about the characteristics of the data than standard univariate procedures (eg, it does not assume equal variance between groups). Next, univariate repeated-measures ANOVAs (general linear model) were performed for each efficacy measure, since the MANOVA group × time interaction was highly significant (P<.005). The sphericity (equal variance assumption) was not applicable to all the follow-up ANOVAs. Therefore we applied the Huynh-Feldt correction to all univariate ANOVAs to adjust for nonsphericity. Post hoc independent t tests were performed on variables (at each time point) if the univariate ANOVA group × time interaction was significant. Safety/adverse symptom data were subjected to a separate repeated-measures ANOVA. In addition, comparisons of the frequency of endorsement of individual adverse symptom scale items between groups were performed with Fisher exact tests. The α level for primary efficacy and safety analyses was set at .05. For secondary measures we adopted a Bonferroni-adjusted α of .01. All tests performed were 2-tailed. Efficacy and safety analyses were performed on the ITT population (n = 47) (defined as all patients who took at least 1 dose of medication and who received at least 1 postbaseline assessment), using last observation carried forward imputations of data. We have reported our data in the text as mean scores ± SD, and where relevant, effect sizes indices (with h indicating differences between proportions).21
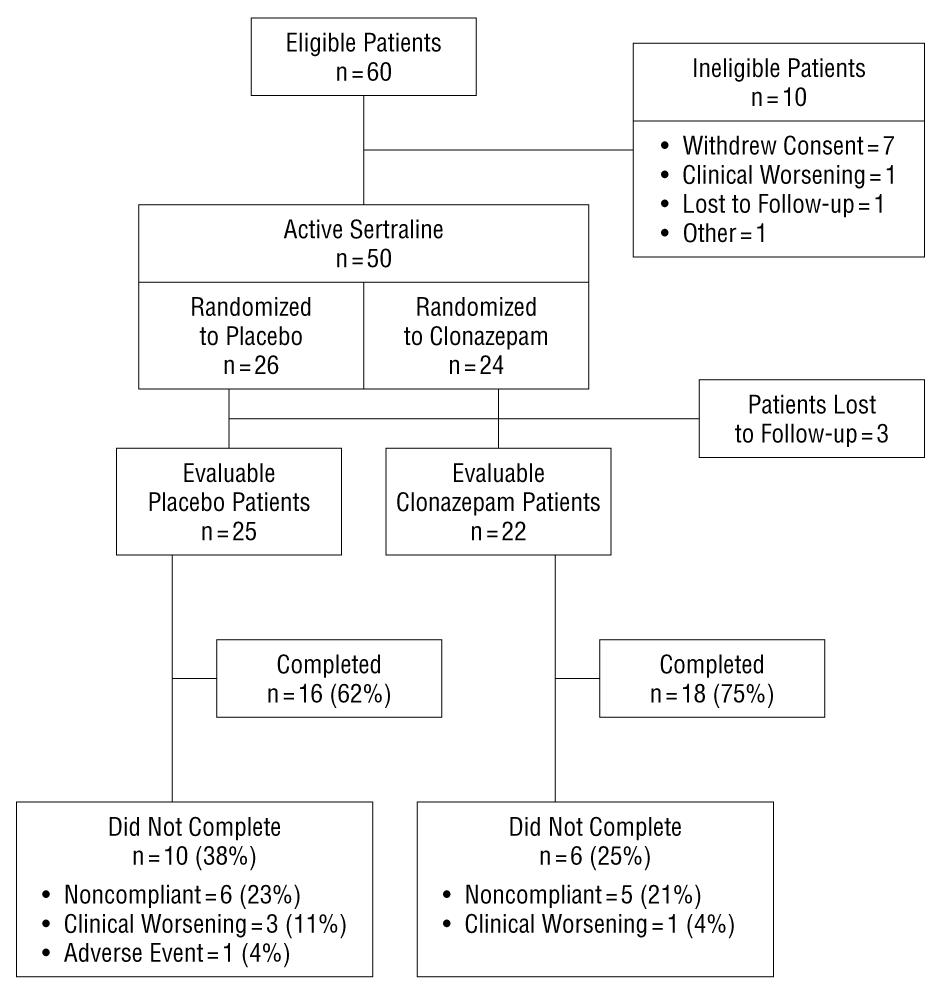
Sixty patients were eligible for the study and were screened accordingly (Figure 1). Of these, 50 were randomized in the protocol. Forty-seven patients formed the ITT population. Table 1 presents the similar demographic and baseline clinical characteristics of the 2 ITT study groups.
Thirty-four of the 50 randomized patients completed the study (68% completion rate) (Figure 1). There were 10 sertraline/placebo group dropouts (38% drop-out rate) vs 6 sertraline/clonazepam dropouts (25% drop-out rate) (h, 0.28; Fisher exact test, P = .37). The most common reason for dropping out in each group was patient noncompliance, including protocol deviations (4 sertraline/placebo vs 1 sertraline/clonazepam patients; Fisher exact test, P = .35), and being lost to follow-up (2 sertraline/placebo vs 4 sertraline/clonazepam patients; Fisher exact test, P = .41). The rates of clinical worsening as a cause of drop-out were similar between groups (3 sertraline/placebo vs 1 sertraline/clonazepam patients; Fisher exact test, P = .61). One patient in the sertraline/placebo group dropped out because of visual hallucinations related to the first dose of medication. A survival curve analysis of the timing and frequency of drop-outs did not differentiate between groups (log-rank statistic, 0.63; df = 1; P = .43). Analysis of the patient adverse symptom data revealed no significant between-group difference for the ITT population (n = 46; ANOVA group × time interaction F3,135 = 0.97; P = .41). In addition, the 2 ITT patient groups had similar patterns of adverse symptoms on the adverse symptom checklist, and only differed significantly on 1 of 29 symptoms (diarrhea) covered by the checklist (Table 2). Three of the 4 sertraline/clonazepam patients reporting diarrhea to be an adverse symptom were noted to have this during the tapering phase of the study (weeks 6, 7, and 8). In general, when side adverse effects occurred, they were of mild to moderate intensity.
There was little deviation from the planned medication-prescribing procedure. Patients were titrated to a sertraline dose of 100 mg/d by the end of week 3 of the study, and remained at this dose level through the end of the study (sertraline/clonazepam group [n = 20] mean ± SD dose at the end of week 3, 100 ± 0 mg vs sertraline/placebo group [n = 24], 98 ± 10 mg; P = .37). Most patients tolerated the fixed dose of clonazepam, 1 capsule 3 times daily (1.5 mg/d clonazepam), until dose tapering began at the end of week 4 (sertraline/clonazepam group [n = 20]; mean ± SD dose at end of week 4, 1.45 ± 0.17 mg vs sertraline/placebo group [n = 24]; mean ± SD dose equivalent, 1.45 ± 0.14 mg; P = .93), and was completed by the end of week 7. The doses for 1 patient in the sertraline/clonazepam group and 1 patient in the sertraline/placebo group were not tapered until the end of week 8. Two patients (1 in the placebo group and 1 in the clonazepam group) were prescribed benzodiazepine medications to use as needed for severe panic during the study. The patient taking clonazepam as needed started taking the medication in week 8 and continued until the end of the study, taking 2 to 3 doses of 0.5 mg lorazepam per week as needed during this period. The patient in the placebo group began taking alprazolam as needed during week 5, taking one 0.25-mg tablet every other week until the end of the study.
There was a marked difference in response rates by the end of week 1 of treatment, with 9 (41%) of 22 patients in the sertraline/clonazepam group responding to treatment vs 1 (4%) of 25 patients in the sertraline/placebo group (h, 0.99; Fisher exact test, P = .003) (Figure 2). This difference was not apparent at the end of week 2 (P = .56). However, there was a between-group difference at the end of week 3, with 14 (63%) of 22 patients in the sertraline/clonazepam group responding vs 8 (32%) of 25 in the sertraline/placebo group responding (h, 0.6; Fisher exact test, P = .05). There were no group differences in response status at the end of weeks 4 (P = .77), or 12 (P = .5), or at any other time during the study. A responder analysis of a subgroup of patients (n = 32; 14 in the sertraline/clonazepam group and 18 in the sertraline/placebo group) who had not received any psychotropic medications in the 6-month period prior to the beginning of the study, revealed a similar finding to the main ITT analysis. At the end of week 1, 5/14 patients (36%) in the sertraline/clonazepam group vs 0 in the sertraline/placebo group responded (h, 1.29; Fisher exact test, P = .01), while at the end of week 3, 10 (71%) of 14 in the sertraline/clonazepam group responded vs 4 (22%) of 18 in the sertraline/placebo group (h, 1.03; Fisher exact test, P = .01).
The MANOVA analysis conducted on all parametric efficacy measures found a highly significant main effect of time (F84,3780 = 5.4; P<.001) and a significant group × time interaction (F84,3780 = 1.45; P<.005). In the follow-up univariate ANOVAs there was a significant group × time interaction for the mean PDSS panic attack scores (item 1), favoring the sertraline/clonazepam group with respect to early efficacy (F8,361 = 3.4; P<.001 (Figure 3). In addition, there was a significant group × time interaction for the mean PDSS sum score favoring the sertraline/clonazepam group (F6,275 = 2.4; P<.02) (Figure 4). However, patient-rated weekly panic attack frequency did not differ between groups (F4,192 = 0.91; group × time interaction, P = .46). Thus, on 3 of 4 primary efficacy measures, there was evidence of superior early efficacy in the sertraline/clonazepam over the sertraline/placebo group. On the secondary efficacy measures there were no significant differences between groups (the group × time interaction terms were as follows: for the HAM-A, F6,250 = 1.2, P = .29; for the CRAS, F5,207 = 1.6, P = .17; for the CGI-S, F7,320 = 1.6, P = .13; and for the CGI-I, F6,260 = 1.6, P = .16).
Xem thêm : Brown Rice Benefits
Our findings, based on controlled data, indicate the efficacy and safety of a rapid stabilization treatment approach (coadministration of early clonazepam and sertraline) for patients with moderate to severe panic disorder. This finding is consistent with findings from other groups.3 Intent-to-treat analyses on 3 of 4 primary efficacy measures demonstrated superior early treatment outcomes in the sertraline/clonazepam patients. We did not observe any statistically significant between-group differences on the secondary efficacy measures, possibly related to the relatively small sample size. However, the omnibus MANOVA (which included all continuous primary and secondary measures) yielded highly significant results (P<.005). Previous medication exposure did not seem to account for the superior early acquisition of response status observed in the sertraline/clonazepam group. Clonazepam coadministration did not seem to have a negative effect on longer-term treatment efficacy, since both groups were comparable in their levels of clinical improvement during the clonazepam dose taper and at study end. In contrast to our earlier work,2 our results suggest not only evidence of rapid stabilization, but also evidence of maintenance of treatment gains during and after benzodiazepine dose taper. Our results may be attributable to the use of the longer-half-life agent, clonazepam, a more gradual benzodiazepine taper (3 weeks vs 2 weeks in our previous work), the improved capacity of SSRIs over tricyclic agents to retain clinical efficacy during benzodiazepine discontinuation, or to a combination of these factors.
The drop-out and safety data suggest that benzodiazepine discontinuation symptoms and other medication adverse effects were generally well tolerated. Adverse effects were of a similar pattern and frequency between groups and were comparable to those observed in the published sertraline/panic disorder literature.10,16,17 The main exception was the symptom of diarrhea, which was more pronounced in the clonazepam-treated patients, and most likely to occur during dose tapering. Since the adverse events checklist we used samples several symptoms of benzodiazepine withdrawal (insomnia, tinnitus, nausea, diarrhea, restlessness, sweating, headache, concentration problems, tremor) there did not seem to be evidence of major benzodiazepine withdrawal symptoms in the sertraline/clonazepam group. However, we caution that more sensitive and specific instruments for measuring benzodiazepine withdrawal are available, such as the physicians withdrawal checklist,22 and therefore subtle withdrawal symptoms may not have been reliably detected with the assessment procedures we employed. The strategy of commencing patients on a fixed dose of clonazepam with gradual upward titration of sertraline was generally well tolerated; very few patients needed to have their benzodiazepine dose lowered due to oversedation.
There are several limitations of the study that should be mentioned. Since we conducted a single-site study on a moderately small sample size, it is conceivable that our findings are attributable to sample or center bias. The sample size may help explain the lack of efficacy findings observed in our secondary measures. Regarding the possibility of center bias, we are reassured to some extent by the emerging and convergent data of other groups. Nonetheless, our findings need to be confirmed in a larger trial to establish the usefulness of this therapeutic strategy. A carefully conducted single- or dual-center follow-up trial may produce a cleaner data set (with less variability) than a traditional multicenter trial. Another aspect of the data, which was unexpected, was the loss of the significant between-group difference on response status by the end of week 2, and then recovery of this difference by the end of week 3. This seemed to be related to a favorable early response to sertraline/placebo treatment (end of week 2), which was not fully established until the end of week 5 (Figure 2), or may have been owing to a disproportionate number of patients in the sertraline/clonazepam group having some activation effects from the SSRI titration (masked by clonazepam), leading to a temporary slowing in the rate of improvement of this group at the end of week 2 (Figure 2). Finally, we failed to observe significant efficacy results with the patient panic diary estimation of weekly panic attack frequency. Although the pattern of the results was similar to that found with the clinician PDSS scale, marked variability in the patients report of panic attacks, coupled with our smaller sample size, contributed to this result. Patient-rated panic records are prone to high variability across subjects, ranging from 0 to infinity, leading to low statistical power.10,16,23 In addition, the lack of consistency between the clinician and patient-panic assessment may partly relate to the fact that the PDSS panic item combines the rating of both limited and full panics.
We conclude that early coadministration of clonazepam and sertraline seems to be a safe and clinically useful strategy to stabilize moderate to severe panic disorder. Follow-up work should incorporate quality of life, work/productivity, and treatment cost measures, to more completely evaluate the cost/benefit ratio of this treatment strategy, and should further explore the optimal timing of benzodiazepine application.
Accepted for publication January 22, 2001.
This work was supported by grants K-08 MH-01322 and R01 MH-58657 (Dr Goddard), and grant MH-30929 (Drs Goddard and Charney), from the National Institute of Mental Health, Bethesda, Md; the Department of Veterans Affairs National Center for Posttraumatic Stress Disorder, West Haven, Conn (Dr Charney); the Connecticut Department of Mental Health and Addiction Services, Hartford; and by an unrestricted educational grant from Pfizer Inc, New York, NY.
Presented as an oral new research presentation at the American Psychiatric Association annual meeting, Chicago, Ill, 2000, May 18, 2000.
We thank the staff of the Yale Anxiety Clinic & Program for their contributions to this work: Kathy Morrissey, BS, Sarah Saiano, Nina Jaynes, Katie Shobe, PhD, Roy Money, MS, Gale Banks, MSN, Nancy T. Ryan.
Corresponding author: Andrew W. Goddard, MD, Yale Anxiety Clinic, 100 York St #2J, New Haven, CT 06511 (e-mail: andrew.goddard@yale.edu).
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on November 26, 2024 1:02 pm