Abstract
Introduction
Direct current (DC) cardioversion of atrial fibrillation to sinus rhythm carries a risk of systemic embolism.1-5 In patients with atrial fibrillation lasting for >48 h or with unknown duration, the conventional approach is to provide anticoagulation for 3-4 weeks prior to and for at least 4 weeks after cardioversion.6 Direct current cardioversion without prolonged oral anticoagulation may be performed in patients with atrial fibrillation duration below 48 h where the risk is considered low or due to acute illness. In a recent study, the risk of thromboembolic complications was high in certain subgroups of patients when no oral anticoagulation was used after cardioversion of acute atrial fibrillation.7 Therefore, it is imperative that better estimates of safety are provided for DC cardioversion of atrial fibrillation. In the present study, we examined a nationwide cohort of 16 274 patients undergoing DC cardioversion of atrial fibrillation discharged from hospitals in Denmark between 2000 and 2008. The purpose was to compare the risk of hospitalization or death due to thromboembolism in patients receiving oral anticoagulant therapy with patients undergoing DC cardioversion without oral anticoagulant coverage.
Methods
Databases
All Danish citizens have a unique personal registration number which enables cross-linkage of nationwide data concerning hospitalization, death, and drug use. Since 1978 the Danish National Patient Registry has registered all hospital admissions in Denmark. Each admission is registered with one primary and, if appropriate, one or more secondary diagnoses using the International Classification of Diseases (ICD-10). Since 2000 all DC cardioversions have been registered and coded according to the Danish National Board of Health classifications of treatment procedures during hospitalization. The Danish Register of Medicinal Product Statistics holds information regarding all prescriptions [coded according to the Anatomical Therapeutic Chemical (ATC) classification system] filled in Denmark since 1995. The registry also includes information on the date of dispensation, strength, and quantity of the drug dispensed. All pharmacies are required by Danish legislation to provide information that ensures complete and accurate registration.8,9 The Danish Cause of Death Registry contains data concerning immediate, contributory, and underlying causes of death classified using ICD-10.
Study population
From the Danish National Patient Registry, we identified all Danish residents with a first-time hospitalization for DC cardioversion for atrial fibrillation between 1 January 2000 and 31 December 2008. The procedure codes identified were: external DC cardioversion (code BFFA0), synchronized DC cardioversion (code BFFA01), and synchronized DC atrial defibrillation (code BFFA04). Only patients with an index or history diagnosis of atrial fibrillation or atrial flutter (ICD-8 code 427.93-94 and ICD-10 code I48), aged 18 years or older, and discharged alive were included.
Comorbidities and thromboembolic risk assessment
Comorbidities of congestive heart failure, hypertension, diabetes mellitus, thromboembolism including ischaemic stroke, transitory ischaemic attack, and peripheral artery embolism, vascular disease including myocardial infarction, peripheral artery disease, and aortic plaque, and bleeding episodes including gastrointestinal bleedings, intracranial bleedings, bleedings in the urinary tract, and bleedings in the airways within 180 days from index admission were determined from the registries as previously described.10,11 All diagnosis and medication codes used for the analyses are listed in the online supplementary material (see Supplementary material online).
Scores of CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischaemic attack), and CHA2DS2-VASc (adding vascular disease, age 65-75, and female sex) were calculated. Identification and validation of the CHADS2 and CHA2DS2-VASc scores in our population have been described in detail previously.11,12
Oral anticoagulant therapy
The Danish Register of Medicinal Product Statistics was used to identify all prescriptions filled of oral anticoagulant therapy; warfarin or Coumadin (ATC code B01AA0). To only consider patients exposed if treated, for every prescription dispensed the applicable exposure status was calculated for each patient. In brief, the daily dosage was estimated for up to seven consecutive prescriptions. This enabled us to calculate treatment regimen at any given time from 90 days within index admission for DC cardioversion and throughout 360 days after discharge. Hence, patients were only deemed exposed when receiving therapy and their expousre status was continually updated.13
Exposure groups
Two exposure groups were defined: the ‘oral anticoagulant therapy group’ and the ‘no oral anticoagulant therapy group’.
Concomitant and post-cardioversion medical therapy
Prescriptions filled of digoxin, amiodarone, class 1C antiarrhythmics, sotalol, beta-blockers without sotalol, non-dihydropyridine calcium-channel blockers, unfractionated heparin (UFH) or low-molecular weight heparin (LMWH), and aspirin within 180 days before index admission were identified and classified as concomitant medical therapy. Prescriptions filled of aspirin within 360 days after discharge were identified. All medication codes used are listed in the online supplementary material (see Supplementary material online).
Endpoints
Outcome measures were hospitalization or death from thromboembolism. A thromboembolic event was defined as an ischaemic cerebral event (ischaemic stroke (ICD-10 code I63), transient ischaemic attack (ICD-10 code G45), unspecified stroke (ICD-10 code I64), or systemic arterial embolism (ICD-10 code I74). Only the first thromboembolic event after DC cardioversion was included in the outcome analysis. Procedure and diagnoses codes used for defining the population, comorbidity, and ortcome are shown in the appendix Table.
Statistical analysis
Xem thêm : Blog
The patients were followed for 360 days after discharge. To assess the timing of thromboembolism, the 360 days of follow-up time was stratified in time intervals of 30 days. Baseline patient characteristics for each exposure group are presented as percentages or means with standard deviations (SDs). Differences in baseline characteristics between patients were assessed by χ2 tests (categorical variables) and the Kruskal-Wallis test (for age). The crude incidence rates per 100 patient-years were assessed in time intervals of 30 days and for the total 360 days of observation, respectively. Adjusted risks were analysed using Cox proportional-hazard regression models. Patients receiving oral anticoagulant therapy were used as reference and the models were adjusted for age, gender, year of index admission, thromboembolic risk factors comprising the CHA2DS2-VASc score, bleeding, and concomitant medical treatment. Model assumptions were tested and found valid unless indicated otherwise. Patients were followed until the first thromboembolic event and censored when dying from causes other than the pre-specified endpoint or passing the end of the observational period (360 days or 31 December 2008). All statistical calculations were performed with the SAS statistical software package version 9.2 (SAS Institute Inc.).
Ethics
The Danish Data Protection Agency approved this study (No. 2008-41-2685), and data were made available at the individual level such that specific individuals could not be identified. For this reason, retrospective ‘register’ studies do not require ethical approval in Denmark.
Results
Characteristics of the study patients
A total of 16 441 patients were admitted for first-time DC cardioversion of atrial fibrillation during 2000-08. Of these, 16 274 (99%) were alive at discharge and included in the study. There were small differences between treatment groups with respect to age, gender, and other thromboembolic risk factors. The mean CHADS2 score was 1.9 and 2.0 for patients with and without oral anticoagulant therapy, respectively (Table 1). Patients with oral anticoagulant therapy received more rate-limiting therapy, while concomitant rhythm control therapy and aspirin therapy were equally distributed (Table 1).
Follow-up data
The mean duration of the follow-up time was 331.8 (SD ± 86.8) days and complete follow-up was achieved in all patients. For patients with no oral anticoagulant therapy, the cumulative incidence of post-cardioversion prescription claims of oral anticoagulant therapy were 27.9 and 46.7% at 30 and 360 days of follow-up, respectively. 89.6% of the patients with prior oral anticoagulant therapy had a prescription refilled within 360 days. Aspirin use within 360 days was 29.8 and 37.0%, for patients with and with no oral anticoagulant therapy, correspondingly. Three hundred and sixty (2.2%) had a thromboembolic complication, 301 (1.8%) were ischaemic stroke, transient ischaemic attack, or systemic arterial embolism, and 59 (0.4%) were unspecified stroke. Twenty-three (0.1%) experienced thromboembolic death. A total of 648 (4.0%) patients died during follow-up.
Thromboembolism within 30 days—primary results
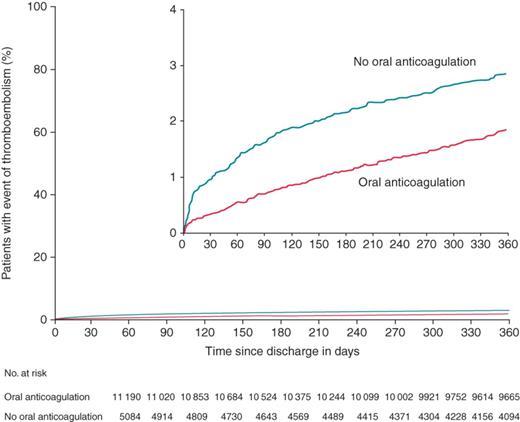
For both treatment groups, the thromboembolic incidence rate was highest in initial 30 days of follow-up, 4.00 and 10.33 per 100 patient-years for patients with and with no oral anticoagulant therapy, respectively. In patients with no oral anticoagulant therapy the hazard ratio (HR) was 2.25; 95% confidence interval (CI), 1.43-3.53 compared with patients with oral anticoagulant therapy (Table 2 and Figure 1). Patients with no prior and no subsequent oral anticoagulant therapy were at high risk for thromboembolic events, HR of 2.47 (95% CI 1.49-4.27). The risk was low in patients with no prior and initiated oral anticoagulant therapy after cardioversion (Figure 2).
Thromboembolism within 360 days of follow-up
The total number of thromboembolic events within 0-360 days occurred in 216 patients with oral anticoagulant therapy (1.84 per 100 patient-years), and in 144 patients with no oral anticoagulant therapy (3.18 per 100 patient-years) corresponding to a HR of 1.87; 95% CI, 1.51-2.32 in the no oral anticoagulant therapy group (Table 2 and Figure 1).
Risk factors and subgroups analyses
Age, previous thromboembolism, and re-hospitalization for atrial fibrillation were significant risk factors of thromboembolism. Risk for thromboembolism increased for patients with high-risk scores (Table 3A and B). Though, among all subgroups the relative benefit of oral anticoagulant therapy remained stable. Notably, thromboembolic risk stratification by the CHADS2 and CHA2DS2-VASc scores did not change the results. The HR in patients with no oral anticoagulant therapy was 2.21; 95% CI, 0.79-6.77 and 2.40; 95% CI, 1.46-3.95 within 0-30 days with CHA2DS2-VASc score 0-1 and CHA2DS2-VASc score 2 or more, respectively. Finally, there was no significant efficacy of aspirin therapy for thromboembolic protection (HR was 0.76; 95% CI, 0.56-1.04 and 0.88; 95% CI, 0.67-1.11 within 0-30 and 0-360 days, respectively).
Discussion
The present study showed that in >16 000 patients discharged after a first-time DC cardioversion for atrial fibrillation, the patients who were not receiving oral anticoagulant therapy had >50% increased risk of thromboembolism within 1 year. Within 30 days we found an almost 2.5-fold increase in risk, indicating a hazardous association between DC cardioversion without oral anticoagulant coverage and thromboembolism. Notably, patients with no anticoagulation therapy were younger, had lesser comorbidity, and lower CHADS2 and CHA2DS2-VASc scores and therefore perceived to have lower thromboembolic risk.
We could not evaluate the reason for omission of oral anticoagulation and the reason may multifactorial, e.g. presumed short duration of atrial fibrillation, clinical emergence, or lack of guideline adherence. The lower thromboembolic risk status in the no oral anticoagulation group suggests that our results were not driven by selection bias. In our study, age and previous thromboembolism were independent risk factors for thromboembolism. In the recent study by Airaksinen et al., age was also a significant risk factor after cardioversion of acute cardioversion without anticoagulation. Importantly, their study also showed that the risk increases with increasing conventional risk factors for thromboembolism.7 Our study support that oral anticoagulant therapy is needed peri-procedural and after DC cardioversion and that long-term therapy should be based on conventional risk factors. Transoesophageal echocardiography is used prior to cardioversion in Denmark, but not commonly and generally in patients presumed to have duration of atrial fibrillation above 48 h.14,15 The value of transoesophageal echocardiography was demonstrated in the ACUTE trial. The importance of oral anticoagulation after DC cardioversion was also highlighted. Point estimates for the risk ratio for mortality, ischaemic stroke, and all embolic events were worse with the transoesophageal echocardiography-guided approach. The embolic events all occurred in patients in atrial fibrillation or with subtherapeutic international normalized ratio (INR) at the time of the embolic events.14 The results of the current study must be weighed against the data supporting current practices of cardioversion of atrial fibrillation without oral anticoagulation. The evidence is very limited. Weigner et al.16 reported that in patients presenting with atrial fibrillation estimated to have lasted <48 h, the likelihood of cardioversion-related thromboembolism is low. From two cardiac centres where pharmacological or DC cardioversion were done, no thromboembolic complications were observed in 107 patients. In line with the observations in the current study, pathological studies have indicated that a finding of thrombi with transoesophageal echocardiography is not uncommon. By using transoesophageal echocardiography, Stoddard et al.13 found atrial thrombi in 14% of patients with atrial fibrillation that lasted <72 h.
Xem thêm : Is Green Tea Good for the Spleen?
An important factor is the inaccuracy related to the clinical estimation of presumed duration of atrial fibrillation. One study in patients who had pacemakers showed that >90% of atrial tachyarrhythmia events documented by the pacemaker were not perceived by the patient, even in those believed to have symptomatic arrhythmia.17
The risk of thromboembolism is also related to other factors than duration of atrial fibrillation. Direct current cardioversion results in the development of temporal depression of left atrial and left appendage function, ‘atrial stunning’, a condition that promotes the formation of new thrombi that later can become dislodged when atrial contraction is restored. Evidence of the formation of new thrombi after cardioversion is well documented in transoesophageal echocardiography studies.18,19 One analysis pooling data from 32 studies and included 4261 patients found 92 (2%) embolic events within 18 days, and 98% of these were observed within 10 days following cardioversion.20 These data support our observations and they are particularly intriguing in the light of our study, in which patients without prior anticoagulant therapy after cardioversion were unlikely to receive oral anticoagulant therapy. The importance of oral anticoagulant therapy after DC cardioversion was demonstrated in our study, where the risk was low in patients initiating oral anticoagulant therapy after cardioversion. The low risk in patients with prior but no subsequent oral anticoagulant therapy could reflect prolonged coverage after treatment interruption.
The implication of the current study is that oral anticoagulant therapy is needed, particularly after DC cardioversion. Moreover, long-term therapy should be based on conventional risk factors as recommended in guidelines.6
The current era of introduction of new oral anticoagulant therapies without the need of close monitoring of anticoagulation might make oral anticoagulation a more feasible alternative in most patients. Recent data support cardioversion with the new oral anticoagulants.21,22,23
Limitations of the study
There are limitations to this study related to the observational nature and the limited data available from registers. Some of the diagnoses used for baseline characteristics may have low accuracy. Atrial fibrillation and thromboembolism diagnoses have been validated previously, e.g. atrial fibrillation had a positive predictive value of 97-99%.24,25 A high positive predictive value (88-97%) of all stroke diagnoses has been found in the Danish Patient Registry. The positive predictive value of ischaemic stroke was 97% and haemorrhagic strokes only comprised 5.8% of the unspecified strokes.26 Even higher positive predictive value of stroke has been found in atrial fibrillation patients possibly because stroke severity is worse in patients with atrial fibrillation.27 While the risk was high in patients not anticoagulated, this study can only hypothesize that the risk would be reduced with anticoagulation. Furthermore, we have not addressed the bleeding risk associated with oral anticoagulation, so direct recommendations on changes in overall treatment strategy based on these data alone would not be appropriate. Finally, the quality of oral anticoagulant therapy through information on INR and time in therapeutic range were not available in the registers used for this study.
Conclusion
Direct current cardioversion for atrial fibrillation without oral anticoagulation is associated with a high risk of thromboembolism. Notably, the risk is high in the initial period after cardioversion, indicating a hazardous association between DC cardioversion without anticoagulation and thromboembolism. Our results support the current practice with oral anticoagulant after DC cardioversion. Long-term therapy should be based on conventional risk factors.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
This study was funded by research grants from the Department of Cardiology, Copenhagen University Hospital Gentofte, Denmark (Hansen). The sponsor of the study had no role in study design, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest: none declared.
References
Nguồn: https://buycookiesonline.eu
Danh mục: Info
This post was last modified on December 7, 2024 7:52 am